
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Acute liver failure is a rare but life-threatening condition characterized by the rapid deterioration of liver function, often in individuals without preexisting liver disease. According to L.M. Martínez-Martínez et al., 2024, it accounts for approximately 2,000 to 3,000 cases annually in North America. The acute liver failure drug pipeline is gaining momentum, driven by the increasing demand for effective therapeutics and growing research efforts. According to the acute liver failure pipeline analysis by Expert Market Research, a surge in novel therapies, including regenerative and anti-inflammatory agents, is expected to accelerate market growth in the coming years, addressing unmet medical needs.
Major companies involved in the acute liver failure pipeline analysis include Beijing Continent Pharmaceutical Co, Ltd., and Shenzhen Zhongke Amshenn Pharmaceutical Co., among others.
Leading drugs currently in the pipeline include F573, VS-01, and MSC-EVs, among others.
With an increased clinical trials of hepatoprotective agents, growing focus on regenerative therapies, and rising investments in targeted biologics development, the acute liver failure drug pipeline is expanding.
The Acute Liver Failure Pipeline Analysis Report by Expert Market Research gives comprehensive insights into acute liver failure therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for acute liver failure. The acute liver failure report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The acute liver failure pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with acute liver failure treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to acute liver failure.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Acute liver failure (ALF) is a rare but life-threatening condition characterized by the rapid deterioration of liver function in individuals without pre-existing liver disease. It typically occurs within days or weeks and can result from viral hepatitis, drug toxicity (commonly acetaminophen overdose), autoimmune disease, or metabolic disorders, leading to coagulopathy and encephalopathy due to the liver's inability to detoxify the blood.
Acute liver failure treatment focuses on stabilizing the patient, managing complications, and addressing the underlying cause. In severe cases, liver transplantation remains the most effective and life-saving intervention. In March 2024, resmetirom showed promising outcomes in the acute liver failure treatment pipeline by improving fibrosis and metabolic profiles in MASLD patients, potentially slowing disease progression and reducing transplant needs.
Acute liver failure (ALF) is a rare but serious condition, with an annual incidence of 2,000 to 3,000 cases in the United States. It accounts for approximately 4-5% of all liver transplants. In developed countries, paracetamol overdose is the leading cause, with an incidence of 5.5-6.2 cases per million annually. In contrast, viral hepatitis and traditional medicines are more prominent causes in developing countries. The etiology remains unknown in many pediatric and adult cases.
This section of the report covers the analysis of acute liver failure drug candidates based on several segmentations, including:
By Phase
By Drug Class
By Route of Administration
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II at 56%, covers a major share of the total acute liver failure clinical trials, indicating strong mid-stage development. Phase I follows with 28%, showcasing steady early-stage innovation. Phase III holds 8%, while both early phase I and phase IV represent the remaining share. This balanced progression supports robust pipeline growth and promises future advancements in acute liver failure treatment.
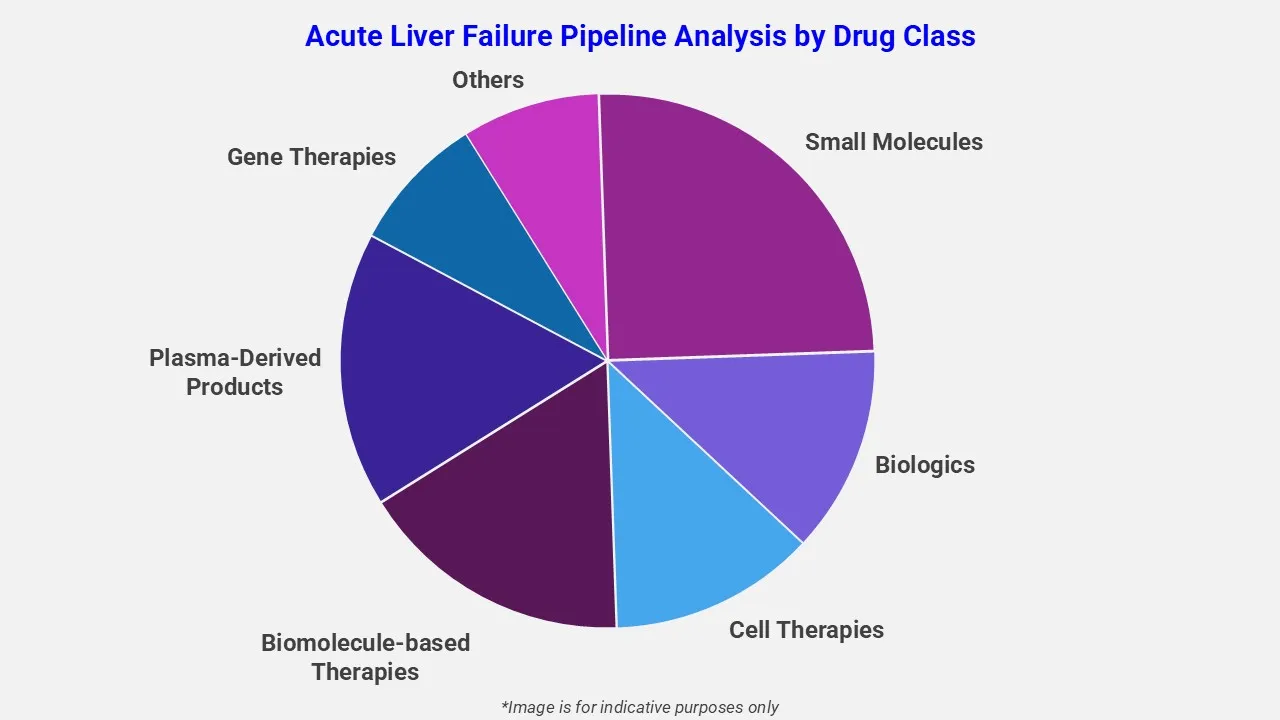
The drug molecule categories covered under the acute liver failure pipeline analysis include small molecules, biologics, cell therapies, biomolecule-based therapies, plasma-derived products, gene therapies, and others. The acute liver failure report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for acute liver failure.
Regenerative therapies are emerging as a promising drug class in the treatment of acute liver failure. For instance, miroliverELAP®, a bioengineered liver assist product, is currently under Phase 1 investigation. It consists of a MIRO-001 graft and an extracorporeal blood circuit, designed to temporarily support liver function for up to 48 hours, allowing time for native liver recovery or transplant evaluation.
The EMR report for the acute liver failure pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed acute liver failure therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in acute liver failure clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for acute liver failure. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of acute liver failure drug candidates.
F573 for Injection is being evaluated by Beijing Continent Pharmaceutical Co., Ltd. in a randomized, double-blind, placebo-controlled Phase II trial for treating acute and acute-on-chronic liver failure. This novel caspase inhibitor targets hepatocyte apoptosis to reduce liver damage. The study is assessing F573’s safety and pharmacokinetics across different liver injury types, with dosing regimens determined based on earlier trial phases and aiming for completion by late 2026.
VS-01, developed by Versantis AG, is currently being evaluated in a Phase 2 study to assess its efficacy, safety, and tolerability in adults with acute-on-chronic liver failure (ACLF) and ascites. This liposomal, intraperitoneal formulation works by capturing and removing ammonia and other harmful metabolites from the abdominal cavity. By restoring metabolic balance and reducing systemic toxicity, VS-01 is aiming to improve organ function when used alongside standard care.
MSC-EVs, sponsored by the Third Affiliated Hospital, Sun Yat-Sen University, is in Phase 1 clinical study. It is evaluating the safety and tolerability of mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) in acute or acute-on-chronic liver failure after liver transplantation. The study is administering a single dose of 10E10 MSC-EV particles post-transplant. MSC-EVs are showing promise as a cell-free therapeutic alternative, offering regenerative and immunosuppressive effects without the risks associated with whole-cell therapies.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Acute Liver Failure Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for acute liver failure. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into acute liver failure collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share