
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
The global rapid test kit market was valued at USD 46.99 Billion in 2025. The market is anticipated to grow at a CAGR of 9.10% during the forecast period of 2026-2035, with the values likely to reach USD 112.27 Billion by 2035. The market growth is driven by the increasing demand for point-of-care (POC) diagnostics. Patents are focused on advanced technologies such as PCR, immunoassay, and HPLC, among others.
Rapid test kits are in high demand due to the rising prevalence of infectious diseases like HIV, malaria, dengue, and COVID-19 as well as chronic illnesses like diabetes and cardiovascular diseases worldwide. In both clinical and residential settings, these kits facilitate early detection, prompt treatment, and disease management.
Rapid test kits are becoming more accurate, sensitive, and quick due to advancements in biosensors, lateral flow assays, and AI-driven diagnostics. Lab-on-a-chip technologies and multiplex testing, which can detect multiple diseases in a single test, are becoming more widely used in healthcare and other fields. Hence, patent applications related to the latest technologies are on the rise.
To enhance disease surveillance and outbreak control, governments and international health agencies (such as the CDC and WHO) are supporting rapid test kits and mass screening initiatives. The adoption is on the rise due to increased funding and regulatory approvals for rapid testing solutions, particularly in environments with limited resources, impacting the patent landscape positively.
The Global Rapid Test Kit Patent Landscape Report provides a comprehensive and in-depth analysis of the patents in this growing industry. The key sections captured in the report for rapid test kit include a thorough examination of the patent portfolios of key players, covering aspects such as the number of patents and types of technologies patented. It includes the latest trends, geographical distribution of patents, top IP player profiles, technological segmentation, and patent valuation associated with rapid test kits. The breakdown of patents by technical segments is provided, giving more clarity on the specific areas of innovation within rapid test kit technologies.
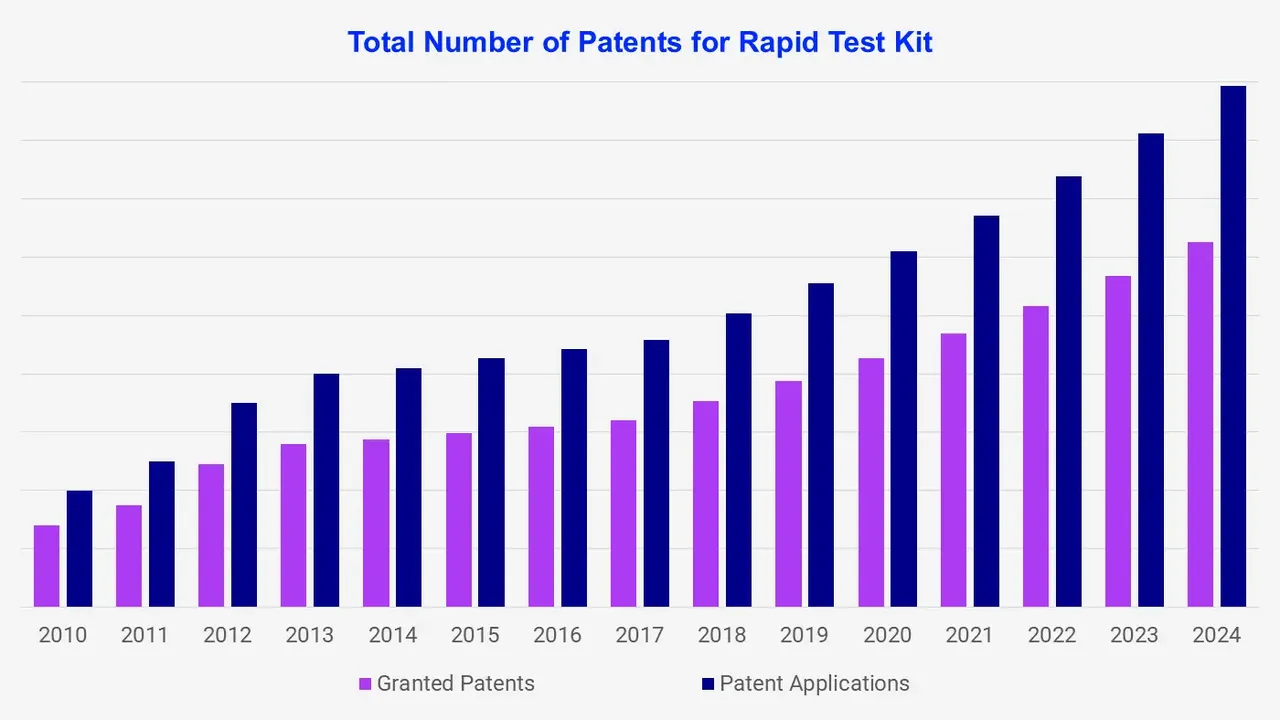
An evaluation and competitive benchmarking of key members of unique patent families are covered in the analysis for rapid test kit technologies. The important parameters have been taken into consideration including IP document, year of application, number of citations, time to expiry, and jurisdiction associated with the rapid test kit.
A comprehensive summary of several patent applications which were filed across different jurisdictions for rapid test kits and their relative value are covered. The analysis will cover the capital in terms of innovation and innovation type.
Detailed analysis of the granted patents across different jurisdictions for rapid test kits and their relative value in the IP are covered in the final report.
Rapid test kits are point-of-care diagnostic instruments made to provide precise and timely results in a matter of minutes without requiring processing in a lab. These kits identify illnesses, infections, or biomarkers in blood, urine, saliva, or swab samples using lateral flow, immunoassay, or molecular detection techniques. In both clinical and home settings, they are frequently utilized for chronic disease monitoring, pregnancy testing, and the detection of infectious diseases (such as COVID-19, HIV, and malaria).
The components of rapid test kits include rapid antigen and rapid antibody. Rapid antigen test kits are point-of-care diagnostic instruments made to identify viral antigens in a sample, usually taken with a throat or nasal swab. These kits are popular for identifying respiratory infections like influenza and COVID-19 because they yield results quickly (within 15 to 30 minutes). Although their sensitivity may be lower than that of molecular tests like PCR, they are inexpensive, simple to use, and appropriate for mass screening.
Technological Advancements in Speed and Test Accuracy is Expected to Boost the Patent Industry Growth
Rapid test kits are becoming faster and more precise due to the technological advancements in test platforms. For instance, Abbott Laboratories was granted a patent for their ID NOW COVID-19 rapid antigen test in 2020. It uses molecular amplification technology to deliver results in as little as 15 minutes. This development pushed the market for quick molecular tests forward by greatly increasing the test's sensitivity and detection speed.
There is an increase in patent activity which focuses on enhancing the performance of rapid test kits. In 2023, 9,658 patents were granted which increased to 10,403 by 2024. Patents covering these technologies are crucial for companies and thus impact the rapid test kit patent landscape.
Increasing Demand for Point-of-Care Testing is Expected to Propel Rapid Test Kit Patent Industry
One of the main factors propelling the market for rapid test kits is the growing demand for point-of-care (POC) diagnostics. These tests provide prompt, convenient, and on-site results, particularly in emergency and remote situations, which minimizes the need for laboratory visits and permits prompt clinical decisions.
The report will cover the following sections in detail:
Analysis by Type
Analysis by Mode of Purchase
Analysis by Technology
The breakup based on the mode of purchase includes over-the-counter rapid test products and professional rapid test products. Consumers can obtain self-use diagnostic kits known as over-the-counter (OTC) rapid test products without a prescription. These tests, which are frequently used to detect HIV, COVID-19, and pregnancy, are easy to use at home and offer fast results (usually in 15 to 30 minutes). They are frequently used for early detection and provide an affordable, convenient means of keeping an eye on medical conditions without having to go to a hospital.
The United States is one of the leading jurisdictions for rapid test kit patents, having around 396,000+ patents. The presence of big companies, well-established healthcare infrastructure, and advanced research and development activities contributes to the regional patent landscape significantly.
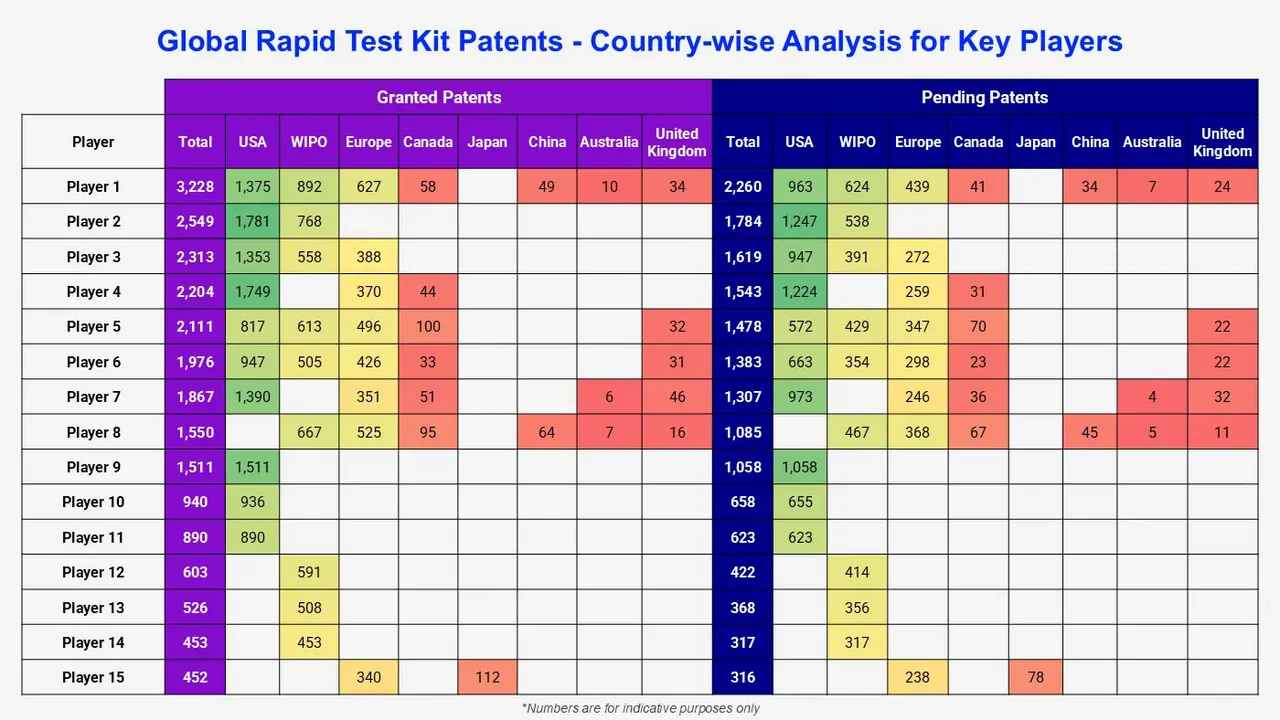
Among the players with rapid test kit patent families, new entrants have been identified, which can be either established companies or startups developing their first technology in the rapid test kit field. Some of the major companies mentioned in this report (a non-exhaustive list) are as follows:
Global medical technology company Becton, Dickinson and Company (BD) specializes in biosciences, diagnostics, and medical devices, such as quick test kits for COVID-19, the flu, and infectious diseases. It was established in 1897 and is based in Franklin Lakes, New Jersey, in the United States. Rapid diagnostic solutions from BD improve disease detection and management while also enhancing point-of-care testing.
B·R·A·H·M·S GmbH is a biotechnology company that specializes in rapid test kits and in-vitro diagnostics, especially for bacterial infections, hormone disorders, and sepsis. B·R·A·H·M·S GmbH was established in 1994 and is a subsidiary of Thermo Fisher Scientific. Its headquarters are in Hennigsdorf, Germany. The business is well-known for its rapid procalcitonin (PCT) tests, which are frequently used in infectious disease and critical care diagnostics.
*Please note that this is only a partial list; the complete list of key players is available in the full report. Additionally, the list of key players can be customized to better suit your needs.*
Other companies are Jordanian Pharmaceutical Mfg, Biolytical Laboratories Inc., and Eachy Biopharmaceuticals Co. Ltd, among others.
The rapid test kit patent report provides information on the intellectual property (IP) position and strategy of key players. This report can help companies and players looking to enter or invest in this field by -
Global Human Immunodeficiency Virus (HIV) Rapid Test Kits Market




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Analysis by Type |
|
|
Analysis by Mode of Purchase |
|
|
Analysis by Technology |
|
|
Analysis by Application |
|
|
Analysis by End User |
|
|
Key Players Mentioned |
|
|
Geographies Covered |
|
Mini Report
One User
USD 2,699
USD 2,429
tax inclusive*
Single User License
One User
USD 4,299
USD 3,869
tax inclusive*
Five User License
Five User
USD 5,799
USD 4,949
tax inclusive*
Corporate License
Unlimited Users
USD 6,999
USD 5,949
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share