
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Cerebral palsy (CP) is the most common motor disability in childhood, comprising a group of permanent disorders that affect movement, posture, and motor function due to non-progressive disturbances in the developing brain. As highlighted in the cerebral palsy pipeline analysis by Expert Market Research, recent population-based studies across different regions report prevalence estimates ranging from 1 to nearly 4 per 1,000 live births or per 1,000 children, underscoring the significant and ongoing global burden of the condition.
Major companies involved in the cerebral palsy pipeline analysis include Novartis, Roche, and others.
Leading drugs currently in the pipeline include FNP-223, Valbenazine, and others.
The growth of the cerebral palsy treatment landscape is driven by the high and persistent prevalence of cerebral palsy worldwide, coupled with increasing focus on early diagnosis and the development of novel therapeutic approaches.
The Cerebral Palsy Pipeline Analysis Report by Expert Market Research gives comprehensive insights into cerebral palsy therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for cerebral palsy. The cerebral palsy report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The cerebral palsy pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with cerebral palsy treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to cerebral palsy.
Read more about this report - Request a Free Sample
Cerebral palsy is a group of permanent neurological disorders caused by non-progressive brain injury or abnormal development occurring during fetal life or early childhood, leading to impaired movement, posture, and motor coordination. The condition often presents in infancy or early childhood and may be accompanied by associated complications such as spasticity, dyskinesia, seizures, and musculoskeletal abnormalities, contributing to a significant lifelong care burden.
The cerebral palsy treatment outlook focuses primarily on symptom management, functional improvement, and quality-of-life enhancement, supported by pharmacological therapies, rehabilitation, and assistive interventions, while emerging pipeline therapies aim to address underlying neurological pathways. Drug development efforts are increasingly targeting movement disorders associated with CP, including dyskinesia and spasticity. Among established therapies, valbenazine, a VMAT2 inhibitor developed by Neurocrine Biosciences, received U.S. Food and Drug Administration approval in April 2017 for movement disorders, and its clinical evaluation in cerebral palsy–related dyskinesia reflects growing regulatory and research momentum. These advancements highlight a gradually evolving pipeline landscape with continued emphasis on improving long-term clinical outcomes in cerebral palsy.
The pipeline continues to evolve as expanding epidemiological understanding supports research prioritization and therapeutic development. According to data referenced by the Centers for Disease Control and Prevention (CDC), cerebral palsy is the most common motor disability in childhood, with recent population-based studies worldwide reporting prevalence estimates ranging from 1 to nearly 4 per 1,000 live births or per 1,000 children. This consistent and substantial prevalence underscores the long-term clinical burden of cerebral palsy across healthcare systems. These epidemiological insights continue to reinforce sustained investment in symptom-targeted treatments and emerging pipeline therapies aimed at improving functional outcomes and quality of life for affected individuals.
This section of the report covers the analysis of cerebral palsy drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
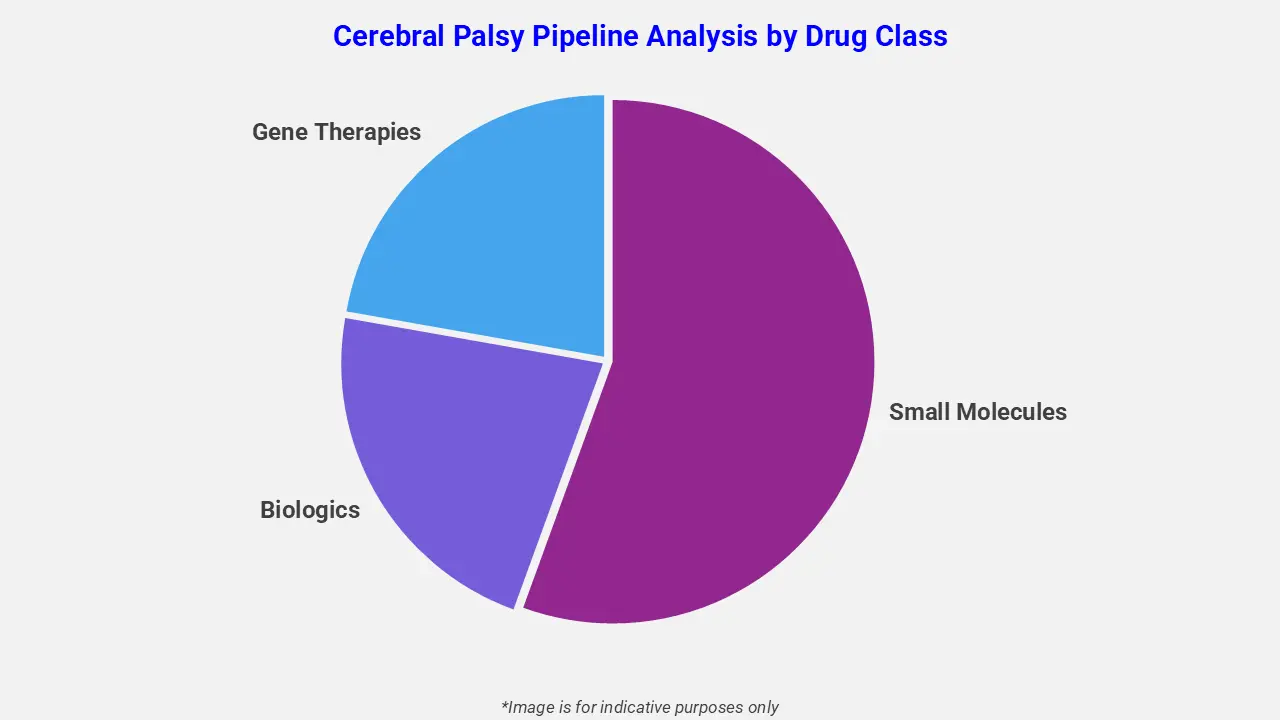
By Drug Class
The cerebral palsy pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II, with 43%, covers a major share of the total cerebral palsy clinical trials. It is followed by phase I at 29.5% and other phases. Overall, this phase-wise distribution highlights a strong focus on mid-stage clinical development, indicating sustained research momentum and a progressing pipeline aimed at advancing effective therapies for cerebral palsy.
The drug molecule categories covered under the cerebral palsy pipeline analysis include small molecules, biologics, and gene therapies. The cerebral palsy report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for cerebral palsy. For example, valbenazine, a small-molecule vesicular monoamine transporter 2 (VMAT2) inhibitor, is being evaluated in late-stage clinical development for the treatment of dyskinesia associated with cerebral palsy.
The EMR report for the cerebral palsy pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed cerebral palsy therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in Cerebral Palsy clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for cerebral palsy. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of cerebral palsy drug candidates.
Valbenazine is being developed by Neurocrine Biosciences, which is sponsoring this Phase 3 interventional study to evaluate its efficacy, safety, and tolerability for the treatment of dyskinesia associated with cerebral palsy. Valbenazine works as a selective vesicular monoamine transporter 2 (VMAT2) inhibitor, reducing presynaptic dopamine release to help control involuntary movements. This investigational oral therapy is being compared with placebo to assess improvements in dyskinetic symptoms, along with overall safety and tolerability. The study is currently active and not recruiting, with an estimated completion timeline between December 2025 and June 2026.
AMX0035 is being developed by Amylyx Pharmaceuticals Inc., which is sponsoring this Phase 2/Phase 3 interventional study to evaluate its efficacy, safety, and tolerability in patients with progressive supranuclear palsy (PSP), a neurodegenerative disorder and form of atypical Parkinsonism. AMX0035 is a fixed-dose combination therapy designed to target key cellular stress pathways implicated in neurodegeneration, intending to protect neurons and slow disease progression. The investigational oral therapy is being compared with placebo to assess its potential impact on disease progression, along with overall safety and tolerability. The study is currently active and not recruiting, with an estimated study completion timeline from April 2026 to November 2029.
UCB0107 (bepranemab) is being developed by UCB Biopharma SRL, which is sponsoring this Phase 1 interventional study to evaluate the long-term safety and tolerability of the therapy in patients with progressive supranuclear palsy (PSP). It is a monoclonal antibody designed to target pathological tau protein, with the aim of reducing tau aggregation and slowing neurodegenerative processes associated with PSP. The study is currently active and not recruiting and focuses on long-term administration to assess safety outcomes in affected participants, with an estimated study completion date of December 2027.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Cerebral Palsy Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for Cerebral Palsy. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into cerebral palsy collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share