
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Cervical Dysplasia is a precancerous cervical epithelial abnormality primarily driven by persistent high-risk HPV infection and detected through routine screening. Epidemiologically, high-grade cervical intraepithelial neoplasia shows incidence rates ranging from 31 to 186 per 100,000 women-years, with prevalence between 0.1% and 2.2% in screened populations, particularly among women aged 25-39 years. According to cervical dysplasia pipeline analysis by Expert Market Research, advancing immunotherapies and biologics aim to address unmet needs beyond surgical management in the evolving global treatment landscape.
Major companies involved in the cervical dysplasia pipeline analysis include IMV, Inc., Inovio Pharmaceuticals, and others.
Leading drugs currently in the pipeline include RG002 injection and others.
The cervical dysplasia pipeline is driven by the growing development of targeted immunotherapies and therapeutic vaccines (e.g., HPV E6/E7-based candidates), non-invasive high-resolution imaging and microendoscopy for precise lesion detection. Novel localized drug delivery systems, enabling precise lesion detection and treatment, accelerating clinical adoption, and differentiating emerging therapies from conventional interventions.
The Cervical Dysplasia Pipeline Analysis Report by Expert Market Research gives comprehensive insights into cervical dysplasia therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for cervical dysplasia. The cervical dysplasia report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The cervical dysplasia pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with cervical dysplasia treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to cervical dysplasia.
Read more about this report - Request a Free Sample
Cervical dysplasia is a precancerous condition often managed through surgical excision and prophylactic HPV vaccination to prevent progression to cervical cancer.
Cervical dysplasia treatment is increasingly evolving beyond surgical excision toward immunotherapy-based and targeted approaches aimed at lesion regression and recurrence prevention. Emerging pipeline therapies focus on managing established CIN2/3 lesions through immune modulation. In April 2024, Yale researchers reported encouraging results using topical imiquimod for CIN2/3 lesions. Delivered as a vaginal suppository, it stimulated local immune responses, promoting lesion regression. When combined with the 9-valent HPV vaccine, the approach showed enhanced efficacy, offering a promising non-surgical treatment option for cervical dysplasia.
Cervical dysplasia, commonly identified as cervical intraepithelial neoplasia (CIN), arises from persistent infection with high-risk human papillomavirus (HPV) types, especially HPV-16 and HPV-18, which are present in the majority of dysplastic lesions. High-grade CIN incidence in screened populations ranges roughly 31-186 per 100,000 women-years, with prevalence of 0.1-2.2 % in screened cohorts; highest rates occur in women aged 25-39 years. Persistent HPV infection markedly increases progression risk from low-grade to high-grade lesions. Globally, HPV prevalence in women with abnormal cytology can exceed 50 %, with the highest rates in high-burden regions. In the United States, about 100,000 women receive treatment for cervical dysplasia annually. Regular screening and HPV vaccination significantly reduce CIN incidence and progression.
This section of the report covers the analysis of cervical dysplasia drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
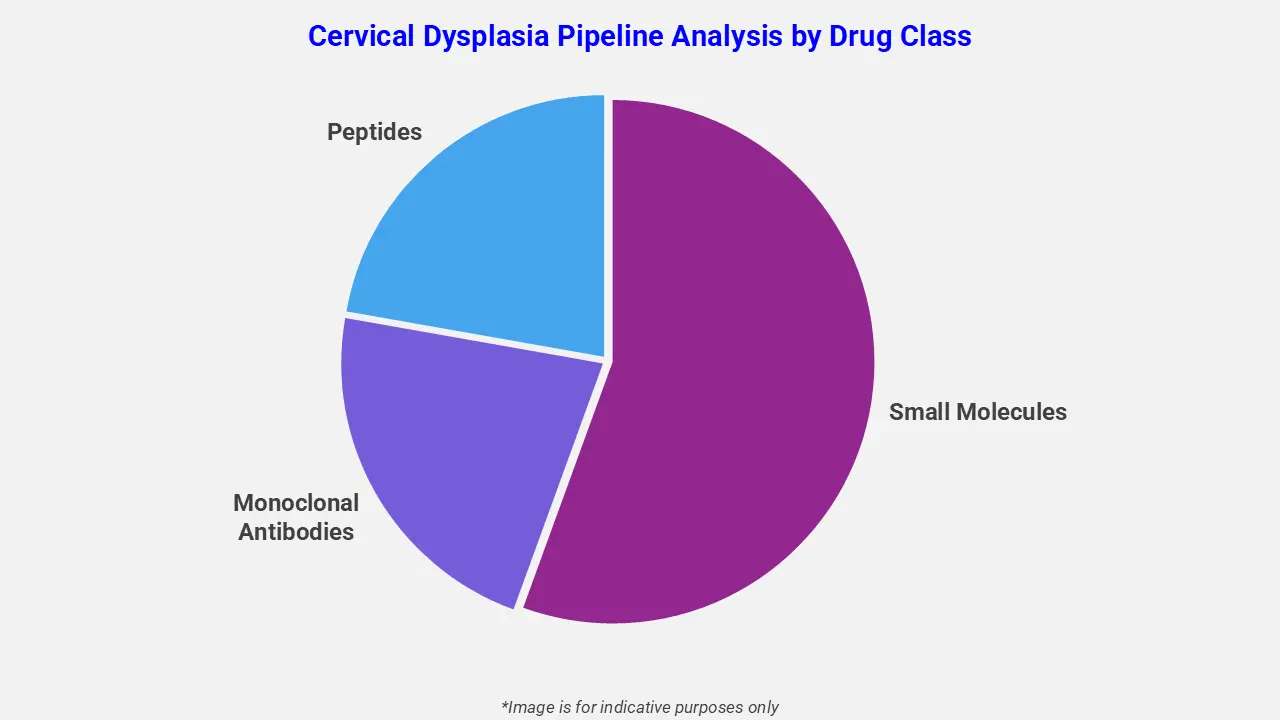
By Drug Class
The cervical dysplasia pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II covers a major share of the total cervical dysplasia clinical trials. Phase 1 represents 31%, while phase 2 accounting for 50%. Late-stage assets remain limited, with Phase 3 at 8% and Phase 4 also 8%, indicating cautious progression toward commercialization across the current clinical research landscape globally.
The drug molecule categories covered under the cervical dysplasia pipeline analysis include small molecules, monoclonal antibodies, and peptides. The cervical dysplasia report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for cervical dysplasia. A notable regulatory milestone occurred in December 2025, when the UK’s MHRA approved the antibody-drug conjugate tisotumab vedotin (Tivdak) for recurrent or metastatic cervical cancer, underscoring growing investment in HPV-related disease treatments beyond prevention. This approval highlights industry momentum toward next-generation biologics and immunomodulators that may eventually influence dysplasia therapeutic strategies.
The EMR report for the cervical dysplasia pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed cervical dysplasia therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in cervical dysplasia clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for cervical dysplasia. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of cervical dysplasia drug candidates.
pNGVL4aCRTE6E7L2 is a DNA-based therapeutic vaccine, classified under immunotherapeutic vaccines. It encodes HPV16 E6 and E7 antigens, designed to stimulate cellular immune responses that target HPV-infected cervical epithelial cells, thereby preventing progression of cervical dysplasia. The vaccine enhances antigen presentation and cytotoxic T-cell activation. Its development and clinical evaluation are led by academic research groups, supported through collaborations with public health institutions, oncology networks, and government-sponsored cancer research programs worldwide.
RG002 injection is a biological therapeutic vaccine candidate designed to treat HPV-related cervical dysplasia. It belongs to the immunotherapy class and works by stimulating targeted immune responses against HPV oncoproteins expressed in dysplastic cervical cells. By activating antigen-specific T-cell responses, RG002 aims to promote lesion regression and viral clearance. The candidate is being developed by RinuaGene Biotechnology Co., Ltd. in focused on HPV-associated precancerous diseases.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The cervical dysplasia Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for cervical dysplasia. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into cervical dysplasia collaborations, regulatory environments, and potential growth opportunities.
Cervical Dysplasia Epidemiology Forecast
Fibrodysplasia Ossificans Progressiva Pipeline Analysis Report




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share