
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
CNS Lymphoma is a rare, aggressive non‑Hodgkin lymphoma confined to the brain, spinal cord, cerebrospinal fluid or eyes, representing about 3-4% of primary CNS tumors with an annual incidence of ~0.4-0.5 per 100,000 people globally. Prevalence increases with age and in immunocompromised patients, particularly those with HIV/AIDS. CNS Lymphoma pipeline analysis by Expert Market Research highlights ongoing epidemiology trends and unmet needs, with incidence projected to rise in aging populations and a growing pipeline of targeted therapies and clinical studies.
Major companies involved in the CNS Lymphoma pipeline analysis include Nurix Therapeutics, Inc., AVM Biotechnology Inc, and others.
Leading drugs currently in the pipeline include NX-2127, 18F-AV-1451, and others.
A unique market driver for the pipeline is the identification of molecular subtypes and actionable biomarkers (e.g., MYD88, CD79B mutations), enabling precision-targeted therapies. Advances in drug delivery across the blood–brain barrier and increasing use of novel immunotherapies such as CAR-T and bispecific antibodies tailored to CNS penetration are accelerating clinical development and investment interest.
The CNS Lymphoma Pipeline Analysis Report by Expert Market Research gives comprehensive insights into CNS Lymphoma therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for CNS Lymphoma. The CNS Lymphoma report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The CNS Lymphoma pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with CNS Lymphoma treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to CNS Lymphoma.
Read more about this report - Request a Free Sample
The CNS lymphoma pipeline outlook reflects growing innovation in a disease traditionally managed with high-dose methotrexate-based chemotherapy, whole-brain radiotherapy, and consolidation with autologous stem cell transplantation. Limitations related to neurotoxicity and relapse have accelerated the development of targeted therapies. Notably, Bruton’s tyrosine kinase inhibitors are reshaping treatment paradigms. In this context, tirabrutinib has emerged as a key pipeline asset. In May 2025, Ono Pharmaceutical reported positive Phase II PROSPECT study results in relapsed or refractory primary CNS lymphoma, demonstrating meaningful response rates. Earlier approvals and launches in Japan further strengthen its clinical and commercial potential.
Central nervous system lymphoma (PCNSL) is a rare but aggressive subtype of non‑Hodgkin lymphoma. It has an estimated annual incidence of approximately 0.5 per 100,000 persons in the United States, equating to about 1,700 new cases per year. PCNSL accounts for roughly 3% of all primary brain tumors and 2-3% of all non‑Hodgkin lymphoma cases. Immunocompetent patients are typically diagnosed between the ages of 50 and 70, while immunocompromised individuals present earlier. Men are affected more often than women.
This section of the report covers the analysis of CNS Lymphoma drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
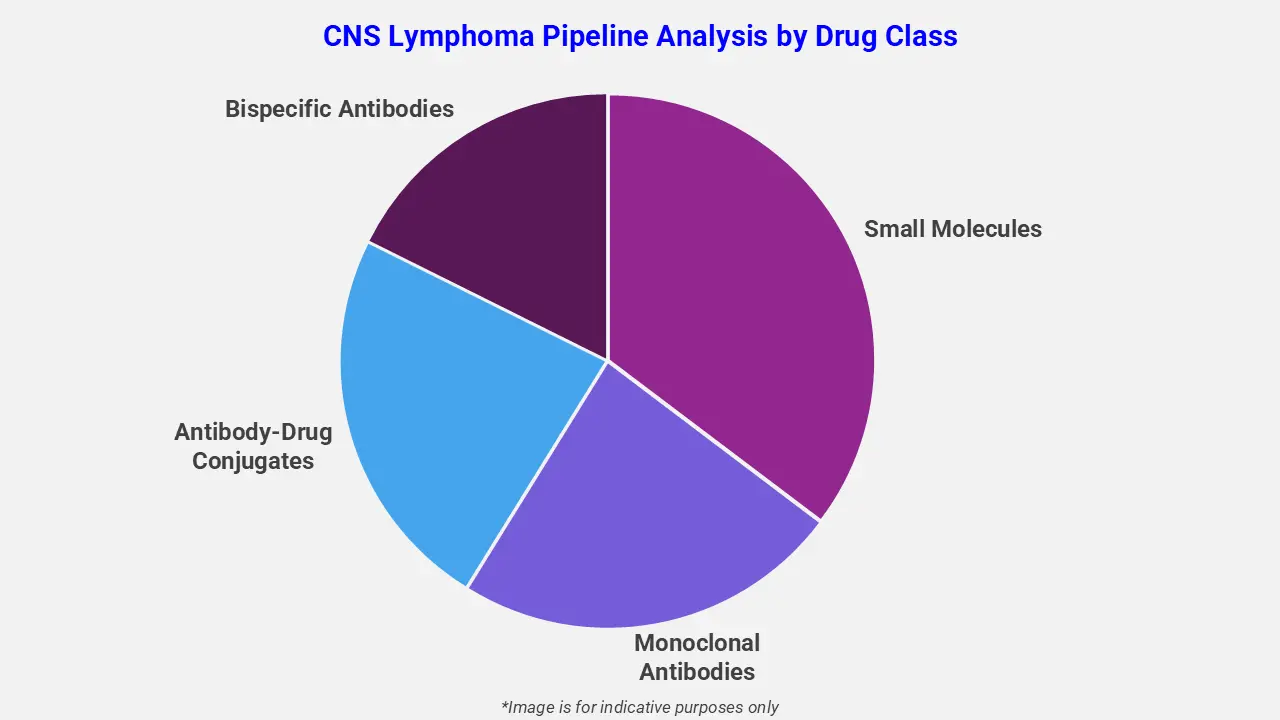
By Drug Class
The CNS Lymphoma pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II covers a major share of the total CNS Lymphoma clinical trials. Phase I accounts for 35% of the pipeline, while Phase III represents 6%, indicating strong clinical activities. This phase distribution highlights a robust early- and mid-stage development landscape, underscoring sustained research momentum and long-term growth potential in the CNS lymphoma therapeutic pipeline.
The drug molecule categories covered under the CNS Lymphoma pipeline analysis include small molecules, monoclonal antibodies, antibody-drug conjugates, and bispecific antibodies. The CNS Lymphoma report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for CNS Lymphoma. Following regulatory approval, tirabrutinib was commercially launched in Japan in May 2020 under the brand name Velexbru®. The launch strengthened the CNS lymphoma pipeline by introducing an oral, brain-penetrant BTK inhibitor, supporting targeted disease management and reinforcing Japan’s leadership in advancing PCNSL-specific therapeutic innovations.
The EMR report for the CNS Lymphoma pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed CNS Lymphoma therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in CNS Lymphoma clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for CNS Lymphoma. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of CNS Lymphoma drug candidates.
AVM0703 is a suprapharmacologic, high‑dose formulation of dexamethasone designed as an immunomodulatory small‑molecule therapeutic with antineoplastic and lymphoablative activity. It works by binding glucocorticoid receptors and triggering mobilization of endogenous bispecific γδ TCR+ invariant TCR+ Natural Killer T‑like cells that can recognize and destroy tumor cells and potentially autoreactive immune cells. AVM0703 is being advanced by clinical‑stage biotech AVM Biotechnology Inc., which is conducting Phase 2 trials in relapsed/refractory Non‑Hodgkin’s lymphoma and exploring broader oncology and autoimmune indications.
CUDC-907, also known as fimepinostat, is an orally available small‑molecule dual inhibitor of phosphatidylinositol 3‑kinase (PI3K) and histone deacetylases (HDACs), classifying it as an antineoplastic epigenetic regulator. It works by simultaneously inhibiting PI3K signaling and HDAC‑mediated chromatin remodeling to induce cancer cell apoptosis and impede survival pathways. Initially developed by Curis, Inc. for hematologic malignancies including relapsed/refractory diffuse large B‑cell lymphoma, it has received regulatory designations and undergone early clinical studies, though development activity has varied over time.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The CNS Lymphoma Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for CNS Lymphoma. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into CNS Lymphoma collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share