
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Congestive heart failure (CHF) is a chronic, progressive cardiovascular condition marked by the heart’s inability to pump sufficient blood to meet the body’s metabolic demands, leading to symptoms such as dyspnea, fatigue, and fluid retention. According to data released by the Heart Failure Society of America, the lifetime risk of heart failure has risen to 24%, with approximately 6.7 million U.S. adults currently living with HF, and the prevalence is projected to reach 8.7 million by 2030. As per the congestive heart failure pipeline analysis by Expert Market Research, sustained pipeline growth is being driven by rising disease burden, increasing healthcare costs, and advancements in pharmacological, biologic, and gene-based therapeutic strategies.
Major companies involved in the Congestive Heart Failure (CHF) pipeline analysis include Novartis, AstraZeneca, and others.
Leading drugs currently in the pipeline include Furosemide, AB-1002, and others.
Rising prevalence driven by an aging population, increasing cardiometabolic risk factors, and escalating healthcare burden is a key growth driver accelerating therapeutic innovation in congestive heart failure.
The Congestive Heart Failure (CHF) Pipeline Analysis Report by Expert Market Research gives comprehensive insights into congestive heart failure (CHF) therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for Congestive Heart Failure (CHF). The congestive heart failure (CHF) report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The congestive heart failure (CHF) pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with congestive heart failure (CHF) treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to congestive heart failure (CHF).
Read more about this report - Request a Free Sample
Congestive heart failure is a chronic, progressive cardiovascular disorder characterized by impaired cardiac pumping capacity, leading to fluid overload, reduced organ perfusion, and recurrent hospitalizations. It arises from diverse etiologies, including ischemic heart disease, hypertension, cardiomyopathy, and metabolic disorders, and represents a major cause of morbidity, mortality, and healthcare expenditure worldwide.
Congestive heart failure treatment continues to rely on established, FDA-approved therapies, with furosemide remaining a cornerstone for the management of fluid overload and acute decompensation due to its potent diuretic effect and ability to relieve congestive symptoms rapidly.
The prevalence of heart failure (HF), including congestive heart failure, is rising significantly across major geographies, reflecting aging populations and improved survival from cardiovascular events. According to the Heart Failure Society of America (HFSA) HF Stats 2025 report, approximately 6.7 million adults aged ≥20 years in the United States are currently living with HF, and prevalence is projected to increase to 8.7 million by 2030, with the lifetime risk now estimated at 24%. Similarly, data from NHS England indicate that around 920,000 people in the UK are living with heart failure, with nearly 200,000 new diagnoses annually; notably, diagnosed cases are expected to almost double by 2040, driven by population aging and improved post–myocardial infarction survival.
This section of the report covers the analysis of congestive heart failure (CHF) drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
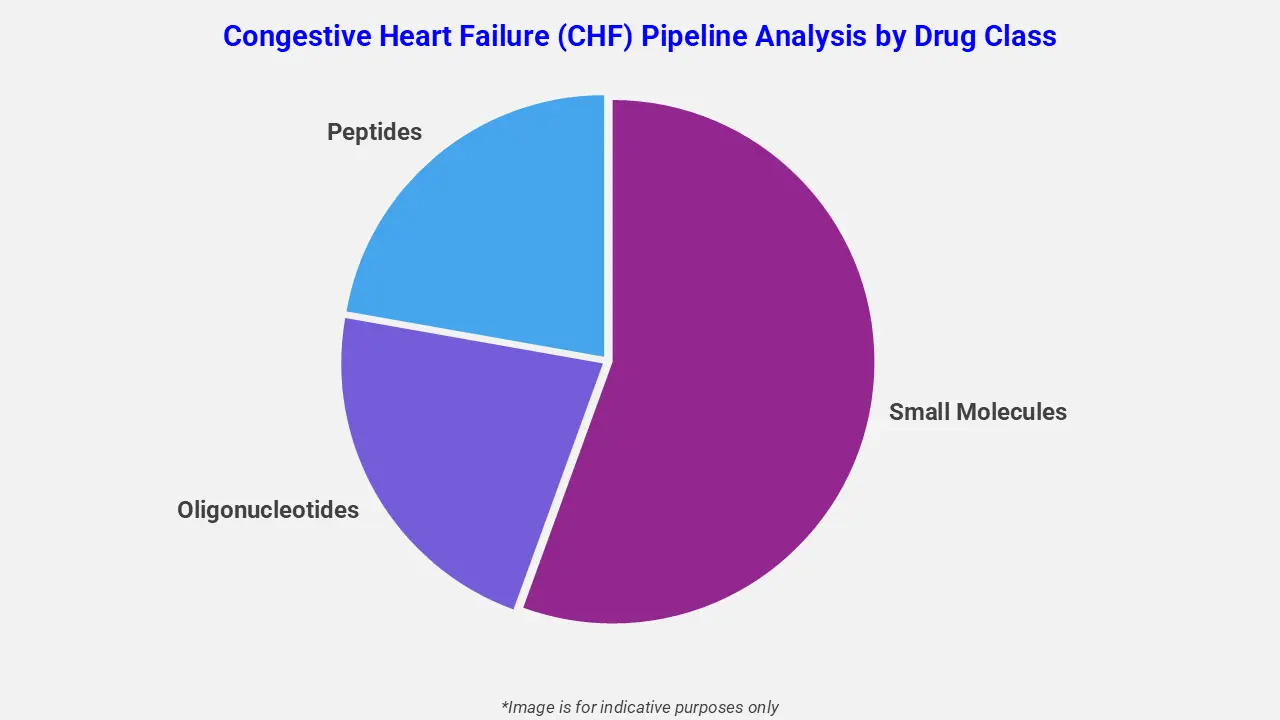
By Drug Class
The congestive heart failure (CHF) pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II, with 44.4%, covers a major share of the total congestive heart failure (CHF) clinical trials. It is followed by phase IV at 33.3% and other phases. This distribution highlights a strong focus on mid-to-late stage clinical validation, reflecting active efforts to optimize existing therapies while advancing promising candidates toward commercialization.
The drug molecule categories covered under the congestive heart failure (CHF) pipeline analysis include small molecules, oligonucleotides, and peptides. The congestive heart failure (CHF) report provides a comparative study of the drug classes for each drug in various phases of clinical trials for congestive heart failure (CHF). For example, furosemide, a well-established small-molecule loop diuretic, is widely used in CHF management and is also being evaluated in ongoing clinical studies for optimized decongestive strategies, underscoring the continued importance of small-molecule therapies within the CHF treatment landscape.
The EMR report for the congestive heart failure (CHF) pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed congestive heart failure (CHF) therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in congestive heart failure (CHF) clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for Congestive Heart Failure (CHF). It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of congestive heart failure (CHF) drug candidates.
AB-1002 is an investigational AAV9-based intracoronary gene therapy being developed by AskBio for patients with congestive heart failure (CHF) due to non-ischemic cardiomyopathy. In the Phase 2 interventional study, AB-1002 delivers a gene encoding a constitutively active inhibitor of protein phosphatase-1 (I-1c) directly to cardiomyocytes. By inhibiting PP1, the therapy enhances SERCA2a activity, improves calcium cycling, and increases myocardial contractility, aiming to restore cardiac function and slow disease progression in NYHA Class III heart failure patients. Study completion is anticipated on December 31, 2030.
Furosemide is a loop diuretic widely used in the management of acute and congestive heart failure (CHF) to relieve fluid overload and congestion. In the Phase 3 interventional study, furosemide 20 mg is being evaluated as part of a decongestive therapy strategy guided by intra-abdominal pressure monitoring and point-of-care ultrasound in patients with acute heart failure, CHF, and cardio-renal syndrome. Furosemide acts by inhibiting the Na⁺-K⁺-2Cl⁻ cotransporter in the thick ascending limb of the loop of Henle, promoting natriuresis and diuresis, thereby reducing preload, venous congestion, and associated renal complications. Study completion is anticipated on December 31, 2026.
Atibuclimab (IC14) is an investigational monoclonal antibody targeting CD14, being developed by Implicit Bioscience for the treatment of acute decompensated heart failure (ADHF). In the Phase 1/2 interventional study, IC14 is designed to modulate the innate immune and inflammatory response implicated in acute heart failure decompensation. By binding to CD14, a key co-receptor in Toll-like receptor signaling, atibuclimab aims to reduce excessive inflammatory activation, improve hemodynamic stability, and mitigate end-organ dysfunction during acute episodes of heart failure. Study completion is expected on January 31, 2026.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Congestive Heart Failure (CHF) Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for congestive heart failure (CHF). It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into congestive heart failure (CHF) collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share