
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Coronary occlusions, critical blockages in the heart’s arteries, are a leading cause of ischemic heart disease and acute myocardial infarction globally. In 2021, approximately 254.3 million people were affected, with 8.99 million deaths worldwide. The coronary occlusions pipeline analysis by Expert Market Research highlights a robust late-stage development landscape, with 28% of studies in Phase 3 and 44% in Phase 4, reflecting significant clinical validation and readiness for broader therapeutic adoption, signaling strong growth potential in the market.
Major companies involved in coronary occlusions pipeline analysis include Verve Therapeutics, Inc., Cyclarity Therapeutics, Inc., and others.
Leading drugs currently in the pipeline include VERVE-102 and others.
The coronary occlusions pipeline growth is being uniquely driven by rising identification of complex lesion phenotypes, including chronic total occlusions and microvascular obstruction. Advances in intracoronary imaging, physiology-guided interventions, and next-generation antithrombotic and device technologies targeting refractory and high-risk occlusive disease subsets.
The Coronary Occlusions Pipeline Analysis Report by Expert Market Research gives comprehensive insights into coronary occlusions therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for coronary occlusions. The coronary occlusions report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The coronary occlusions pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with coronary occlusions treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to coronary occlusions.
Read more about this report - Request a Free Sample
Coronary occlusions, blockages in the heart’s arteries disrupting blood flow, are typically treated with percutaneous coronary intervention (PCI) using drug-eluting stents (DES) to reopen narrowed vessels and prevent restenosis.
Coronary occlusion treatment is evolving with the introduction of device-based innovations that aim to reduce long-term complications associated with permanent implants. Emerging therapies such as drug-coated balloons (DCBs) offer non-metallic alternatives to stents, particularly for small and complex lesions. In August 2025, Boston Scientific initiated the global STANCE trial evaluating the AGENT drug-coated balloon, the only FDA-approved coronary DCB, in de novo coronary lesions. The study targets small vessels, bifurcations, and long lesions, enrolling over 1,600 patients, potentially expanding DCB use beyond in-stent restenosis and reshaping future coronary occlusion treatment paradigms.
Coronary occlusions are a major component of ischemic heart disease (IHD), a condition resulting from blocked coronary arteries that restrict blood flow to the heart muscle. As per the American Heart Association, 254.3 million people were affected globally in 2021, causing 8.99 million deaths that year, making IHD one of the leading causes of mortality worldwide. Age‑standardized IHD death rates were highest in Central Asia, Eastern Europe, and North Africa/Middle East, reflecting significant regional disparities in disease burden and healthcare access. The global IHD prevalence and mortality continue to rise, remaining substantial drivers of cardiovascular health loss, with males generally more affected than females.
This section of the report covers the analysis of coronary occlusions drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
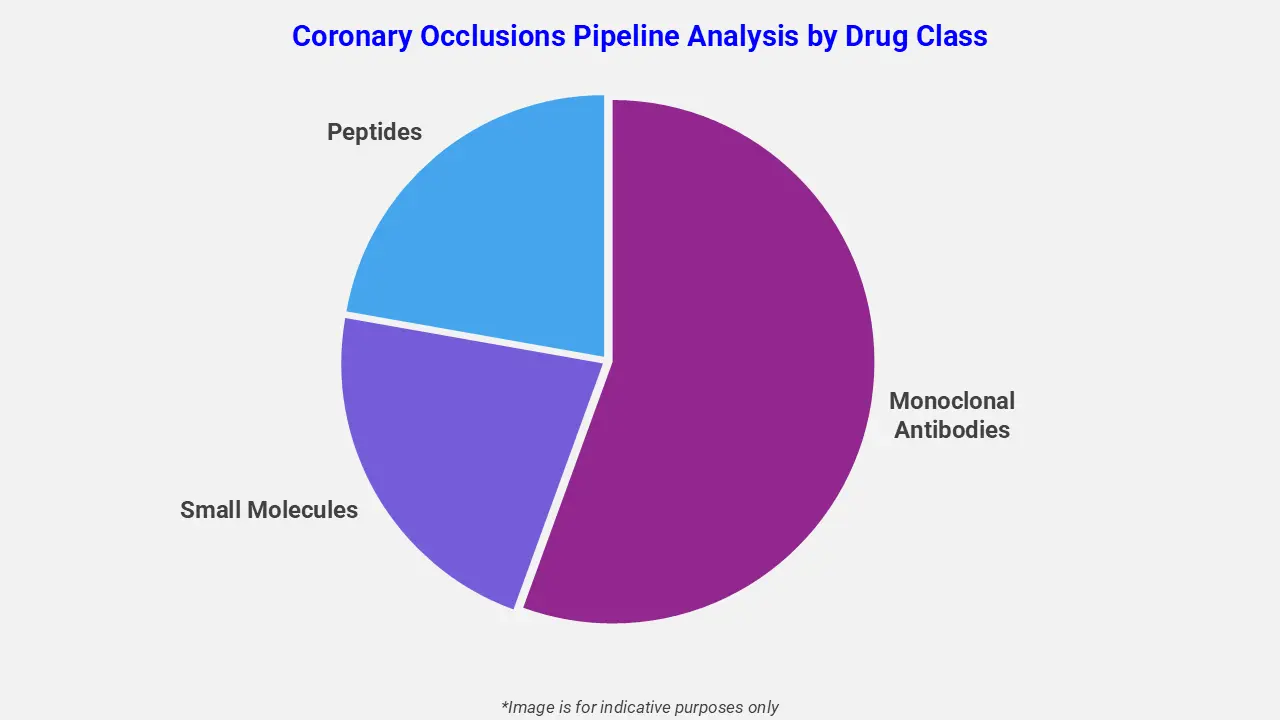
By Drug Class
The coronary occlusions pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II covers a major share of the total coronary occlusions clinical trials. The pipeline comprises 20% of phase II, 28% of phase III, and 44% of phase IV studies, reflecting a strong emphasis on late-stage development. The higher proportion of Phase 3 and 4 trials indicates significant clinical validation and readiness for widespread therapeutic adoption in real-world cardiovascular management.
The drug molecule categories covered under the coronary occlusions pipeline analysis include monoclonal antibodies, small molecules, and peptides. The coronary occlusions report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for coronary occlusions. In July 2025, CorFlow announced FDA approval to initiate the pivotal MOCA‑II IDE trial, evaluating its CoFl system for diagnosing and managing microvascular obstruction (MVO) during primary PCI in STEMI patients. This trial targets improved outcomes after reopening occluded coronary arteries, representing a significant advancement in the coronary occlusions treatment pipeline.
The EMR report for the coronary occlusions pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed coronary occlusions therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in coronary occlusions clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for coronary occlusions. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of coronary occlusions drug candidates.
HeXell-2020 is an investigational small-molecule therapeutic being explored within the cardiovascular space, potentially positioned as a vascular or anti-ischemic modulator. The candidate is designed to influence pathological mechanisms involved in coronary blood flow impairment, such as endothelial dysfunction or thrombosis-related signaling, thereby supporting myocardial perfusion. Early studies suggest a focus on disease-modifying activity rather than symptomatic relief alone. This drug is sponsored by Hexun Biosciences Co., Ltd., with ongoing efforts to advance the molecule through early clinical evaluation.
XC001 is a novel investigational compound, classified as a small-molecule cardiovascular agent, under evaluation for conditions associated with coronary occlusion and ischemic heart disease. The drug is intended to act on molecular pathways linked to vascular inflammation, plaque instability, or myocardial ischemia, aiming to reduce adverse coronary events. Its mechanism is being characterized through translational studies to establish therapeutic relevance in high-risk cardiac populations. XC001 is currently being sponsored by XyloCor Therapeutics, Inc., which is focused on innovative therapies for unmet needs in cardiovascular medicine.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Coronary Occlusions Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for coronary occlusions. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into coronary occlusions collaborations, regulatory environments, and potential growth opportunities.
Acute Coronary Syndrome Pipeline Analysis Report




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share