
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Epidermolysis bullosa (EB) is a rare genetic skin disorder characterized by extreme skin fragility, causing painful blisters and wounds from minor friction or trauma. According to Aaron Tabor et al., 2024, it accounts for around 1 in 18,000 live births globally. The pipeline for epidermolysis bullosa therapeutics is expanding, with a growing focus on gene therapies, protein replacement, and cell-based treatments. Increased research funding and orphan drug designations are driving innovation. According to the epidermolysis bullosa pipeline analysis by Expert Market Research, the treatment landscape is expected to witness significant growth in the coming years due to rising clinical advancements.
Major companies involved in the epidermolysis bullosa pipeline analysis include Krystal Biotech, Inc., Castle Creek Biosciences, LLC., and others.
Leading drugs currently in the pipeline include KB803, AGLE-102, ELK-003, and others.
Pipeline growth for epidermolysis bullosa is driven by increased clinical trials of gene therapies, rising FDA designations for rare disease drugs, and advancements in protein replacement treatments.
The Epidermolysis Bullosa Pipeline Analysis Report by Expert Market Research gives comprehensive insights into epidermolysis bullosa therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for epidermolysis bullosa. The epidermolysis bullosa report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The epidermolysis bullosa pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with epidermolysis bullosa treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to epidermolysis bullosa.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Epidermolysis bullosa is a group of rare genetic disorders that cause the skin to become extremely fragile and blister easily from minor friction or trauma. It occurs due to mutations in genes responsible for skin integrity, particularly those that encode anchoring proteins between skin layers.
Epidermolysis bullosa treatment focuses on wound care, pain management, and infection prevention. Currently, gene therapies and protein replacement therapies are emerging as promising approaches. In May 2023, the U.S. Food and Drug Administration approved Vyjuvek, a topical gene therapy for dystrophic epidermolysis bullosa. Vyjuvek delivers normal COL7A1 gene copies directly to wounds using a modified herpes-simplex virus, promoting healing and skin integrity in affected patients.
According to Aaron Tabor et al., 2024, the prevalence and incidence of epidermolysis bullosa (EB) vary across regions. In England and Wales, the prevalence was 34.8 per million, with 1,200 births recorded from 2002 to 2022. The United States reported a lower prevalence of 8.2 per million live births. Germany and the Netherlands reported incidences of 45 and 41.3 per million live births, respectively. In Iran, 538 cases were documented in 2021. Globally, EB affects approximately 1 in 18,000 live births. These figures highlight the need for improved diagnostics and global data collection.
This section of the report covers the analysis of epidermolysis bullosa drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
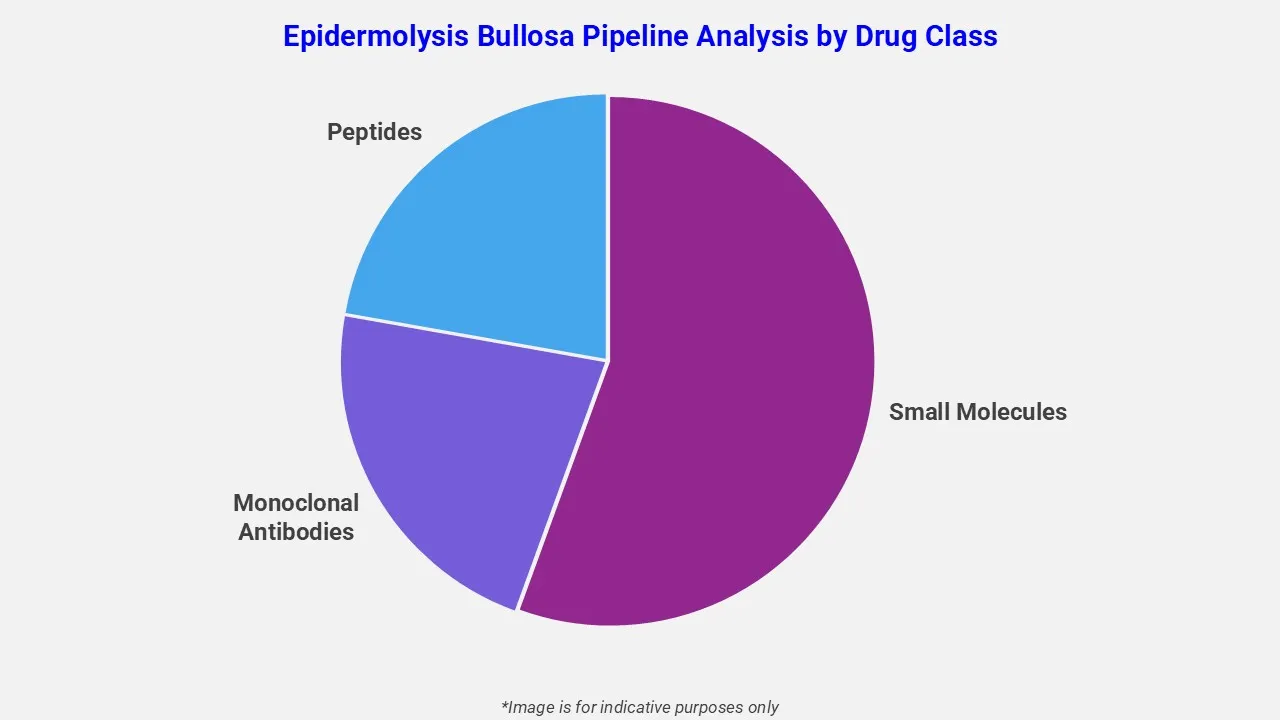
By Drug Class
The epidermolysis bullosa pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration.
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II at 41%, covers a major share of the total epidermolysis bullosa clinical trials. Phase III follows with over 26%, reflecting strong momentum toward advanced clinical validation. Phase I contributes 20.59%, indicating steady research investment. This well-distributed pipeline signals promising advancements for the epidermolysis bullosa treatment landscape.
The drug molecule categories covered under the epidermolysis bullosa pipeline analysis include small molecules, monoclonal antibodies, and peptides. The epidermolysis bullosa report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for epidermolysis bullosa. Topical therapies are gaining momentum in the treatment pipeline for Epidermolysis Bullosa. For instance, FILSUVEZ, a birch triterpene-based topical gel, has been approved for dystrophic and junctional epidermolysis bullosa. It promotes wound healing through anti-inflammatory and skin-repair properties. The therapy is applied directly to partial-thickness wounds and has demonstrated efficacy in pediatric and adult patients during clinical trials.
The EMR report for the epidermolysis bullosa pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed epidermolysis bullosa therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in epidermolysis bullosa clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for epidermolysis bullosa. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of epidermolysis bullosa drug candidates.
KB803 is a redosable gene therapy eye drop being sponsored by Krystal Biotech. It aims to treat and prevent corneal abrasions in patients with dystrophic epidermolysis bullosa (DEB). This therapy is under a Phase 3 trial study, which is assessing the safety and efficacy of KB803. It delivers two copies of the COL7A1 gene to restore type VII collagen in corneal epithelial cells. The therapy is currently being administered in an intra-patient, double-blind, crossover design.
AGLE-102 is an investigational extracellular vesicle (EV) therapy developed by Aegle Therapeutics. It is derived from donor mesenchymal stem cells (MSCs) and is undergoing a Phase 1/2A trial to evaluate its safety and efficacy in healing chronic skin lesions in patients with recessive dystrophic epidermolysis bullosa (RDEB). The study is examining whether multiple EV applications, combined with standard care promote wound closure more effectively than standard care alone.
ELK-003 eye drops, sponsored by Fundación DEBRA Chile, are currently undergoing a Phase 1 pilot study to treat ocular manifestations in patients with junctional and dystrophic epidermolysis bullosa. The study is evaluating the efficacy of ELK-003, a standardized amniotic fluid secretome rich in collagen 7 and laminin-332. This phase is focusing on assessing improvements in corneal integrity and healing by comparing observational and treatment outcomes.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Epidermolysis Bullosa Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for epidermolysis bullosa. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into epidermolysis bullosa collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share