
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Epstein-Barr virus (EBV) is a common human herpesvirus linked to infectious mononucleosis and various malignancies. As per Jean de Melo Silva et al., 2024, EBV globally affects approximately 90-95% of adults, highlighting its widespread prevalence. Current therapeutic strategies focus on antiviral drugs, immunotherapies, and vaccines aimed at controlling viral replication and reducing disease complications. There is a growing emphasis on targeted therapies and novel vaccine development, driven by rising EBV-associated disorders and unmet clinical needs. According to the Epstein-Barr virus pipeline analysis by Expert Market Research, the EBV drug pipeline is expanding rapidly, indicating strong growth potential in the coming years.
Major companies involved in the Epstein-Barr virus pipeline analysis include AstraZeneca, Novartis Pharmaceuticals, and others.
Leading drugs currently in the pipeline include 2LEBV® / 2LXFS®, GP350 CAR-T, EBV gp350-Ferritin Vaccine, and others.
The pipeline shows significant expansion driven by novel antiviral candidates, increasing focus on immunotherapeutic approaches, and rising investments in targeted therapies, indicating strong growth potential over the coming years.
The Epstein-Barr Virus Pipeline Analysis Report by Expert Market Research gives comprehensive insights into Epstein-Barr virus therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for the Epstein-Barr virus. The Epstein-Barr virus report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The Epstein-Barr virus pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with Epstein-Barr virus treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to the Epstein-Barr virus.
Read more about this report - Request a Free Sample
Epstein-Barr virus (EBV) is a widespread herpesvirus that infects over 90% of the global population. It primarily spreads through saliva and can remain latent in B-cells, occasionally triggering diseases like mononucleosis or EBV-driven cancers in immunocompromised individuals. Infection occurs when the virus enters epithelial or immune cells, hijacking cellular machinery to replicate.
Epstein-Barr virus treatment focuses on symptom management and antiviral research, as no specific cure exists. Therapies include immunomodulatory drugs, antiviral agents, and targeted cancer therapies for EBV-associated malignancies, aiming to reduce viral replication and disease progression. In July 2025, the Epstein-Barr virus pipeline featured PARP1 inhibitors, such as talazoparib, showing promise against EBV-driven lymphomas by disrupting the EBNA2/MYC oncogenic axis. This FDA-approved drug significantly reduced tumor growth and metastasis in preclinical models, offering potential for rapid clinical application.
According to Jean de Melo Silva et al., 2024, Epstein-Barr virus (EBV) infects 90-95% of adults globally, reflecting its high prevalence. As per Infectious Disease Advisor, EBV accounts for over 90% of infectious mononucleosis cases worldwide. EBV is strongly linked to nasopharyngeal carcinoma (NPC) and extranodal NK/T-cell lymphoma. Shyh-An Yeh et al., 2024, report that NPC incidence is higher in Asia and North Africa, with age-standardized rates of 1.7–1.8 per 100,000 persons. In China and Taiwan, rates reach 2.1–6.4 per 100,000, with males showing 3.5 times higher incidence. EBV prevalence in non-keratinizing NPC reaches nearly 100%, emphasizing its critical role in pathogenesis.
This section of the report covers the analysis of Epstein-Barr virus drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
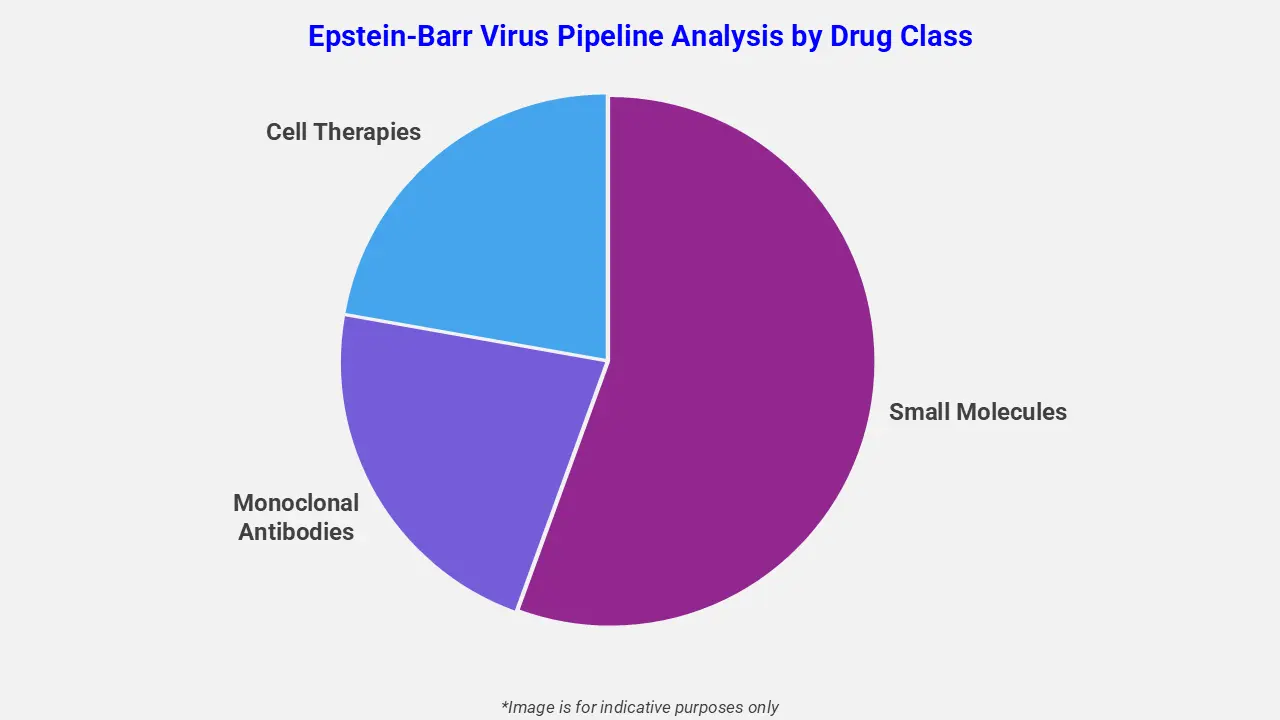
By Drug Class
The Epstein-Barr virus pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase I covers a major share of the total Epstein-Barr virus clinical trials, with 46% of developments, driven by early safety and tolerability studies. Phase II follows with 42%, focusing on efficacy and dosing optimization. This diverse phase distribution highlights a strong pipeline capable of advancing innovation, improving treatment options, and expanding market potential.
The drug molecule categories covered under the Epstein-Barr virus pipeline analysis include small molecules, monoclonal antibodies, and cell therapies. The Epstein-Barr virus report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for Epstein-Barr virus. Antiviral small-molecule therapies targeting Epstein-Barr Virus (EBV) are gaining attention for treating EBV-associated cancers. For instance, VK-2019, a small-molecule EBNA1 inhibitor, is under clinical evaluation for EBV-positive nasopharyngeal carcinoma. Developed at The Wistar Institute, VK-2019 inhibits viral replication, reduces tumor growth, and has shown a favorable safety profile in early human trials, supporting its potential as a targeted EBV therapy.
The EMR report for the Epstein-Barr Virus pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed Epstein-Barr Virus therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in Epstein-Barr Virus clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for Epstein-Barr virus. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of Epstein-Barr virus drug candidates.
2LEBV® and 2LXFS® are investigational drugs sponsored by Labo'Life, currently being studied in a Phase 4, randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov ID NCT04308278). Both drugs are oral capsules, administered sequentially, one capsule per day for six months, to evaluate their effectiveness in reducing asthenia and other symptoms associated with Epstein-Barr virus (EBV) infections. 2LEBV® is designed to target fatigue by modulating the host immune response and supporting viral control, while 2LXFS® is tested in combination to enhance therapeutic outcomes. The study is examining the efficacy, safety, and tolerability of these drugs compared with placebo in adults with chronic or acute EBV infection.
GP350 CAR-T is an investigational cellular therapy targeting EBV-associated lymphoid neoplasms, sponsored by Zhimin Zhai at The Second Hospital of Anhui Medical University. This Phase I/Phase II open-label, single-arm study is examining the safety, tolerability, and preliminary efficacy of GP350 CAR-T in patients with relapse/refractory disease. The therapy involves leukapheresis followed by conditioning chemotherapy and intravenous infusion of CAR-T cells engineered to recognize the EBV envelope glycoprotein GP350, which mediates viral entry via CD21 on B cells. Administered intravenously, GP350 CAR-T is designed to selectively target EBV-infected cells while sparing normal cells, with ongoing post-treatment assessments and long-term follow-up.
EBV gp350-Ferritin Vaccine is an investigational nanoparticle vaccine being developed to prevent Epstein-Barr virus (EBV) infection, sponsored by the National Institute of Allergy and Infectious Diseases (NIAID). This Phase 1 study is examining the safety and immunogenicity of a three-dose regimen administered intramuscularly with a saponin-based Matrix-M adjuvant. The vaccine targets EBV glycoprotein gp350, a key viral surface protein, displayed on ferritin nanoparticles to enhance immune recognition. Healthy adults, both EBV-seropositive and -seronegative, are receiving doses at 0, 1, and 6 months while researchers are monitoring antibody and CD4 T cell responses, local and systemic reactions, and overall tolerability over 12–18 months.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Epstein-Barr Virus Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for Epstein-Barr virus. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into Epstein-Barr virus collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share