
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Ichthyosis is a group of rare genetic skin disorders characterized by chronic dry, scaly skin, significantly impacting patients’ quality of life. Globally, ichthyosis vulgaris affects approximately 1 in 100-250 individuals, while severe syndromic forms like Netherton syndrome are far rarer. The ichthyosis drug pipeline analysis by Expert Market Research highlights emerging disease-modifying therapies, including protein-targeted biologics and gene-based approaches, which aim to correct underlying molecular defects, offering unprecedented opportunities for treatment innovation in this underserved dermatology segment.
Major companies involved in the ichthyosis pipeline analysis include Azitra Inc., BioCryst Pharmaceuticals and others.
Leading drugs currently in the pipeline include BCX17725, and others.
The ichthyosis drug pipeline is propelled by innovative gene- and protein-targeted therapies, such as engineered biologics and KLK5 inhibitors, addressing underlying molecular defects rather than symptoms. Rising investment in rare disease platforms and orphan drug incentives further accelerates development, creating growth opportunities for novel, disease-modifying candidates in a previously underserved dermatology segment.
The Ichthyosis Pipeline Analysis Report by Expert Market Research gives comprehensive insights into ichthyosis therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for ichthyosis. The ichthyosis report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The ichthyosis pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with ichthyosis treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to ichthyosis.
Read more about this report - Request a Free Sample
The ichthyosis treatment pipeline is evolving from purely symptomatic management toward disease-modifying approaches. Currently, standard care primarily relies on topical emollients, keratolytics, and supportive barrier-repair therapies, with no FDA-approved targeted treatments available.
Ichthyosis treatment innovation is now focused on correcting underlying molecular defects. In October 2025, Azitra, Inc. announced positive preclinical results for its ATR-01 program targeting ichthyosis vulgaris. The engineered ATR01-616 strain successfully delivered functional filaggrin through human skin and reduced transepidermal water loss in preclinical models. These findings, presented at BIO-Europe 2025, support progression toward first-in-human studies planned for 2026.
Ichthyosis encompasses multiple genetic skin disorders, with ichthyosis vulgaris being the most common and X-linked ichthyosis the second most prevalent form. X-linked ichthyosis affects males predominantly, with an incidence ranging from 1 in 2,500 to 1 in 6,000 males worldwide and equally affects all ethnic groups. Symptoms appear at birth in about 15-20% of cases, with the remainder developing scaling during early infancy. Although definitive treatment does not exist, these conditions are lifelong and significantly impact quality of life due to chronic dry, scaly skin and associated complications.
This section of the report covers the analysis of ichthyosis drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
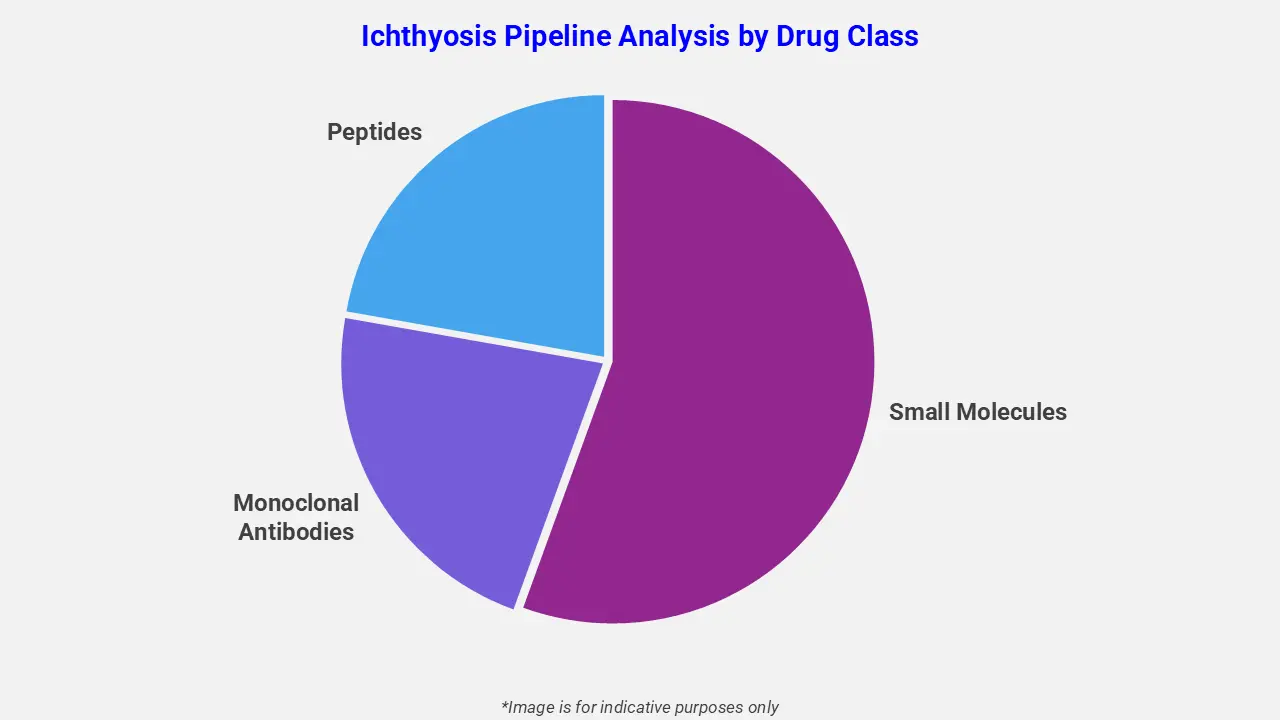
By Drug Class
The ichthyosis pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II dominates with 38% of trials, followed by phase I at 24%, phase III 19%, early-phase I 10%, and phase IV 10%. Overall, this distribution highlights a balanced pipeline with strong mid- to late-stage clinical development activity in ichthyosis therapies.
The drug molecule categories covered under the ichthyosis pipeline analysis include monoclonal antibodies, small molecules, and peptides. The ichthyosis report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for ichthyosis. In August 2025, Quoin Pharmaceuticals reported Q2 2025 results, highlighting positive six-month clinical data from an ongoing pediatric Netherton syndrome study and FDA clearance of a second pivotal trial for QRX003. The company also announced initial positive results in peeling skin syndrome, received Orphan Drug designation in Europe, and earned U.S. Rare Pediatric Disease designation for QRX003.
The EMR report for the ichthyosis pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed ichthyosis therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in ichthyosis clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for ichthyosis. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of ichthyosis drug candidates.
QRX003, 4% Lotion is an investigational topical therapy developed for the treatment of Netherton syndrome, a rare genetic form of ichthyosis characterized by severe skin barrier dysfunction and inflammation. The lotion is designed to deliver a broad-spectrum serine protease inhibitor directly to the skin to help restore barrier integrity, reduce inflammation, and improve clinical symptoms associated with excessive protease activity. The therapy is currently being evaluated in a Phase II/III interventional clinical study assessing safety, tolerability, and efficacy in affected patients. QRX003 is being developed by Quoin Pharmaceuticals as part of its rare dermatology pipeline focused on genetic skin disorders.
BCX17725 is an investigational protein therapeutic biologic designed to address the underlying protein deficiency in Netherton syndrome, a rare genetic ichthyosis. It functions as a KLK5 inhibitor to regulate dysregulated protease activity on the skin, potentially restoring normal barrier function. BCX17725 is in Phase I clinical testing, assessing safety, tolerability, pharmacokinetics, and immunogenicity. BioCryst Pharmaceuticals, a U.S. biotech firm, is developing this candidate as one of its early‑stage rare disease programs.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Ichthyosis Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for ichthyosis. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into ichthyosis collaborations, regulatory environments, and potential growth opportunities.
Ichthyosis Epidemiology Forecast




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share