
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal tract, mainly including Crohn’s disease and ulcerative colitis. According to Devendra Desai et al. (2023), there were 6.8 million global cases of IBD. According to the inflammatory bowel disease (IBD) pipeline analysis by Expert Market Research, therapies are advancing toward targeted biologics and small-molecule treatments. The growing focus on early diagnosis, personalized therapies, and improved safety profiles is expected to drive strong market growth in the coming years.
Major companies involved in the inflammatory bowel disease (IBD) pipeline analysis include AstraZeneca, Mirador Therapeutics, Inc., and others.
Leading drugs currently in the pipeline include MORF-057, SPY001, AZD7798, and others.
The IBD drug pipeline is expanding, driven by rising biologics innovation, growing investment in targeted immunotherapies, and increased clinical focus on next-generation oral small molecules that aim to improve long-term disease control.
The Inflammatory Bowel Disease (IBD) Pipeline Analysis Report by Expert Market Research gives comprehensive insights into inflammatory bowel disease (IBD) therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for inflammatory bowel disease (IBD). The inflammatory bowel disease (IBD) report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The inflammatory bowel disease (IBD) pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with inflammatory bowel disease (IBD) treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to inflammatory bowel disease (IBD).
Read more about this report - Request a Free Sample
Inflammatory bowel disease (IBD) is a chronic gastrointestinal disorder that primarily includes Crohn’s disease and ulcerative colitis. It arises from a combination of abnormal immune responses, genetic predisposition, and environmental factors, leading to persistent intestinal inflammation, tissue damage, and recurrent digestive symptoms that significantly impact patients’ quality of life.
Inflammatory bowel disease (IBD) treatment aims to control inflammation, achieve and maintain remission, and prevent relapses using biologics, immunomodulators, anti-inflammatory drugs, and targeted therapies tailored to patient-specific disease activity. In January 2025, Eli Lilly’s Omvoh (mirikizumab-mkrz) advanced the IBD drug pipeline by demonstrating robust Phase 3 results, delivering sustained clinical remission and meaningful endoscopic healing for adults with moderately to severely active Crohn’s disease, offering a new long-term treatment option.
According to Devendra Desai et al., 2023, there were 6.8 million cases of inflammatory bowel disease (IBD) globally. As per inflammatory bowel disease (IBD) epidemiology data by Expert Market Research, incident cases increased by 88.3% between 1990 and 2021, reaching 4.45 cases for every 100,000 people. According to Gastroenterology Advisor, IBD affects millions in the United States, with prevalence steadily rising. As per Mehmet A Veral et al., 2025, approximately 0.2% of the European population has IBD, with the United Kingdom reporting 540,000 Crohn’s and Colitis cases.
According to Bénédicte Caron et al., 2024, annual incidence rates vary by region, ranging from 10.5 to 46.14 per 100,000 in Europe, 1.37 to 1.5 per 100,000 in Asia and the Middle East, 23.67 to 39.8 per 100,000 in Oceania, 0.21 to 3.67 per 100,000 in South America, and 7.3 to 30.2 per 100,000 in North America. These trends indicate a growing need for innovative therapies in the IBD drug pipeline.
This section of the report covers the analysis of inflammatory bowel disease (IBD) drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
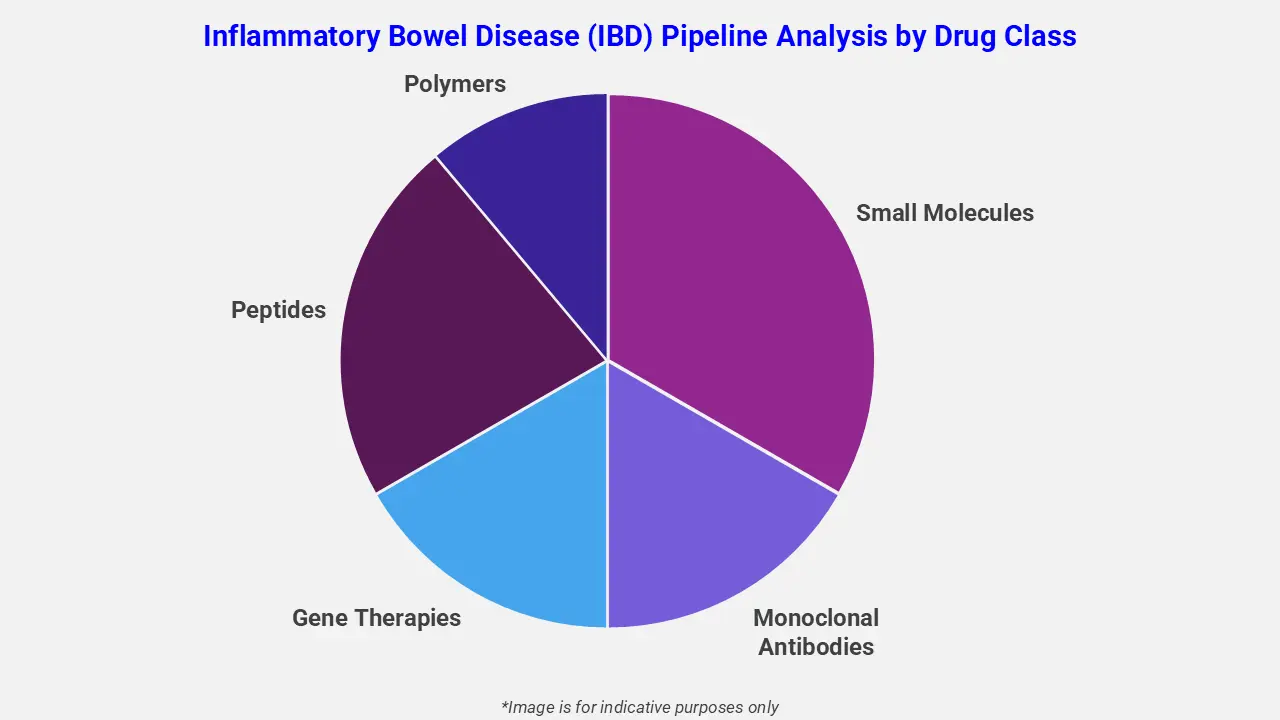
By Drug Class
The inflammatory bowel disease (IBD) pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration.
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II, with 39%, covers a major share of the total inflammatory bowel disease (IBD) clinical trials. It is followed by phase III at 26% and phase I at 16%. Phase IV accounts for 14%. Strong activity highlights robust clinical development, signaling a promising future for innovative IBD therapies and potential market growth opportunities.
The drug molecule categories covered under the inflammatory bowel disease (IBD) pipeline analysis include small molecules, monoclonal antibodies, gene therapies, peptides, and polymers. The inflammatory bowel disease (IBD) report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for inflammatory bowel disease (IBD). Immune-modulating therapies are emerging as promising options in the inflammatory bowel disease (IBD) pipeline. For instance, hAEC-derived extracellular vehicles (EVs) are under clinical investigation for complex perianal Crohn’s disease. These EVs deliver regenerative and anti-inflammatory molecules, mimicking stem cell effects at lower cost. Moreover, novel biologics targeting pro-inflammatory cytokines, including TNF-α and IL-23 inhibitors, are also under evaluation to improve patient outcomes.
The EMR report for the inflammatory bowel disease (IBD) pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed inflammatory bowel disease (IBD) therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in inflammatory bowel disease (IBD) clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for inflammatory bowel disease (IBD). It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of inflammatory bowel disease (IBD) drug candidates.
MORF-057 is an oral small-molecule inhibitor of integrin α4β7, developed by Morphic Therapeutic, Inc., a wholly owned subsidiary of Eli Lilly and Company. The Phase 2b EMERALD-2 study is evaluating its safety and efficacy in adults with moderately to severely active ulcerative colitis. MORF-057 works by selectively inhibiting integrin α4β7, a protein that enables immune cells to enter intestinal tissue and trigger inflammation. By blocking this pathway, the drug is aiming to reduce inflammatory cell migration, lower gut inflammation, and improve IBD symptoms. The study is expected to be completed by August 2026.
SPY001 is an investigational, extended half-life monoclonal antibody developed by Spyre Therapeutics, Inc. and is targeting the α4β7 integrin, a key regulator of lymphocyte trafficking in inflammatory bowel disease. Designed to match the potency and selectivity of vedolizumab, SPY001 is demonstrating a significantly longer ~80-day half-life, enabling quarterly or biannual subcutaneous maintenance dosing following intravenous induction. The ongoing Phase 2 SKYLINE-UC trial is evaluating its ability to reduce inflammation and improve outcomes in moderate to severe ulcerative colitis. The study is likely to be completed in March 2028.
AZD7798 is an emerging oral investigational anti-inflammatory drug developed by AstraZeneca, designed to modulate intestinal inflammatory pathways in Crohn’s disease. It is believed to be working by targeting specific molecular receptors involved in gut inflammation and potentially supporting mucosal healing. This Phase Ib PET study is examining how multiple doses of AZD7798 are altering intestinal uptake of the radioligand [11C]AZ14132516 to assess target engagement. The trial is recruiting 12 participants and is expected to be completed by August 2026, offering early insights into the drug’s biological activity.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Inflammatory Bowel Disease (IBD) Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for inflammatory bowel disease (IBD). It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into inflammatory bowel disease (IBD) collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share