
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Myopia progression is the gradual worsening of nearsightedness, which can lead to severe vision complications if left untreated. According to Gregory Ostrow et al., 2025, the global prevalence of myopia has surged from 22.9% in 2000 to an estimated 34% in 2020 and is projected to reach 50% by 2050. Current therapies include low-dose atropine eye drops, orthokeratology lenses, and specialized corrective spectacles. The growing focus on early intervention, personalized treatment, and innovative drug development is driving market expansion. According to the myopia progression pipeline analysis by Expert Market Research, the treatment landscape is expected to evolve in the coming years.
Major companies involved in the myopia progression pipeline analysis include Ocumension (Hong Kong) Limited, Sydnexis, and others.
Leading drugs currently in the pipeline include OT-101, SYD-101, and others.
The pipeline is expanding rapidly, driven by increased R&D in novel pharmacological therapies, rising pediatric prevalence, and advancements in targeted ocular treatments, supporting robust market growth in coming years.
The Myopia Progression Pipeline Analysis Report by Expert Market Research gives comprehensive insights into myopia progression therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for myopia progression. The myopia progression report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The myopia progression pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with myopia progression treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to myopia progression.
Read more about this report - Request a Free Sample
Myopia progression is the gradual worsening of nearsightedness, where the eye elongates, causing distant objects to appear blurry. It typically occurs in children due to genetic factors, increased screen time, and reduced outdoor exposure, accelerating vision deterioration over time.
Myopia progression treatment includes low-dose atropine eye drops, orthokeratology lenses, and specialized optical interventions designed to slow eye elongation and reduce long-term visual complications. In July 2025, Santen Pharmaceutical launched Ryjunea® in Germany, marking the first EU-approved low-dose atropine eye drop for pediatric myopia. Ryjunea® provides ophthalmologists with a clinically validated option to slow myopia progression and protect children’s vision, reflecting a significant advancement in the myopia progression treatment pipeline.
According to Gregory Ostrow et al., 2025, the global prevalence of myopia increased from 22.9% in 2000 to 34% in 2020 and is projected to reach 50% by 2050, affecting nearly 5 billion people. As per Mukharram M. Bikbov et al., 2024, high myopia (≤ −6.00 dioptres) may affect about 10% of the population. Children and adolescents, particularly in East Asia and urban areas, show the highest prevalence, with factors including age, sex, parental myopia, indoor activities, and reduced outdoor time.
This section of the report covers the analysis of myopia progression drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
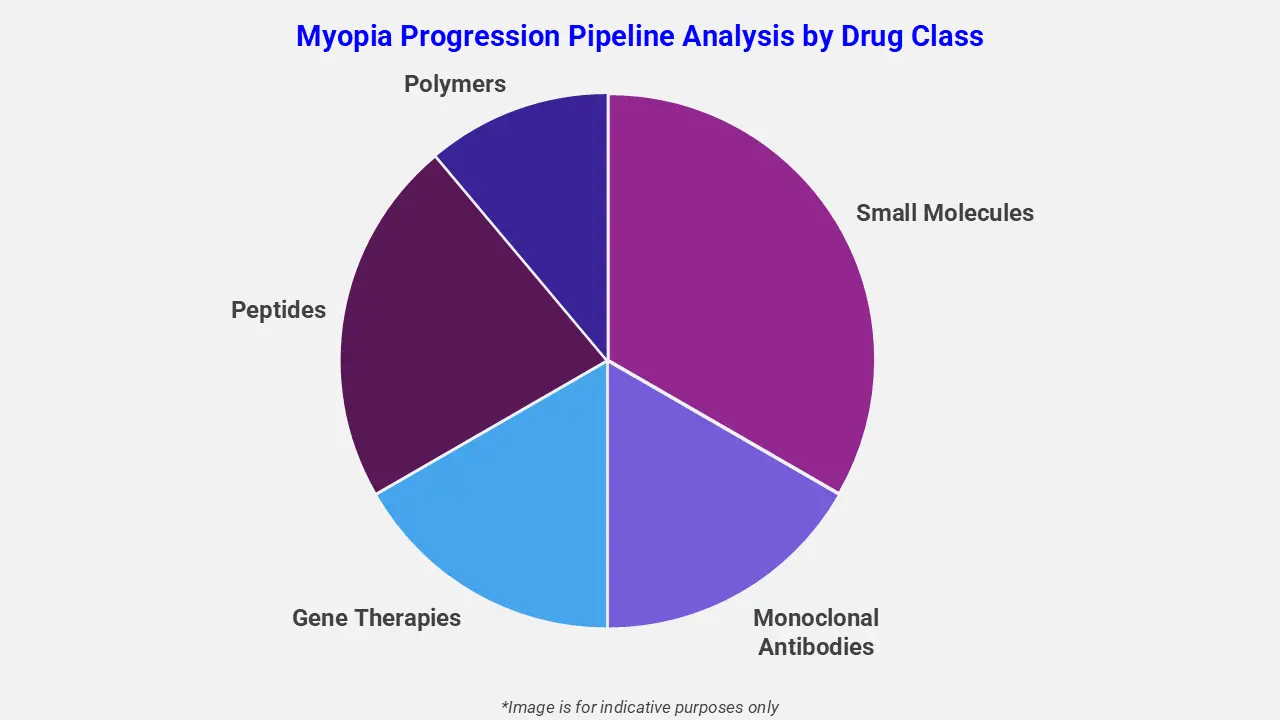
By Drug Class
The myopia progression pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration.
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase III, with approximately 62%, covers a major share of the total myopia progression clinical trials. It is followed by Phase II at 31%, and Phase I at 6%. This distribution indicates a robust late-stage development, with several therapies nearing potential market approval. Advancements in these phases could significantly enhance treatment options, offering more effective and accessible solutions for managing myopia progression. Such developments are poised to positively impact patient outcomes and market dynamics.
The drug molecule categories covered under the myopia progression pipeline analysis include small molecules, monoclonal antibodies, gene therapies, peptides, and polymers. The myopia progression report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for myopia progression. Oral pharmacological therapies are emerging as a promising approach for myopia progression control in children. For instance, ND10, a solid oral tablet, increases the biomechanical strength of the sclera and prevents axial elongation. Only phase-3 ready drug in advanced development, ND10 has demonstrated a 75% reduction in myopia progression, with a compelling safety profile in over 1,200 pediatric patients.
The EMR report for the myopia progression pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed myopia progression therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in myopia progression clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for myopia progression. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of myopia progression drug candidates.
OT-101 (Atropine Sulfate 0.01%) is being sponsored by Ocumension (Hong Kong) Limited and is undergoing a Phase III clinical trial to evaluate its safety, tolerability, and efficacy in slowing myopia progression in pediatric subjects. This randomized, double-masked, placebo-controlled study is examining the effects of once-daily bedtime eye drops over three years. OT-101 works by relaxing eye muscles and is being investigated for its potential to reduce worsening nearsightedness in children.
SYD-101 is a low-dose atropine sulfate eye drop developed by Sydnexis to slow the progression of pediatric myopia. The Phase 3 STAR trial is examining its efficacy in children aged 3 to 14 years with myopia ranging from 0.5 D to 6.0 D. Administered nightly, SYD-101 is designed to deliver optimal stability, superior drug activity, and comfort. The study is evaluating confirmed myopic progression, aiming to reduce future ocular complications such as cataracts, glaucoma, and retinal detachment.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Myopia Progression Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for myopia progression. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into myopia progression collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share