
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Nasopharyngeal cancer is a rare malignancy that originates in the nasopharynx, the upper part of the throat behind the nose. According to the International Agency for Research on Cancer, it accounted for approximately 120,416 new cases globally in 2022, representing 0.6% of all cancer diagnoses. With growing interest in targeted and immuno-oncology therapies, the nasopharyngeal cancer drug pipeline is expanding steadily. According to the nasopharyngeal cancer pipeline analysis by Expert Market Research, therapeutics such as Toripalimab, a PD-1 inhibitor, show promising results in advanced stages. Increased R&D investments and rising awareness are expected to drive significant growth in the coming years.
Major companies involved in the nasopharyngeal cancer pipeline analysis include MediLink Therapeutics (Suzhou) Co., Ltd., Sichuan Baili Pharmaceutical Co., Ltd., and others.
Leading drugs currently in the pipeline include YL201, HLX43, APG-5918, and others.
The nasopharyngeal cancer drug pipeline is expected to grow due to rising clinical trials of targeted therapies, increasing immunotherapy approvals, and expanding research collaborations among key pharmaceutical companies.
The Nasopharyngeal Cancer Pipeline Analysis Report by Expert Market Research gives comprehensive insights into nasopharyngeal cancer therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for nasopharyngeal cancer. The nasopharyngeal cancer report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The nasopharyngeal cancer pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with nasopharyngeal cancer treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to nasopharyngeal cancer.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Nasopharyngeal cancer is a rare malignancy that begins in the nasopharynx, the upper part of the throat behind the nose. It typically develops from epithelial cells and is strongly associated with Epstein–Barr virus infection, genetic susceptibility, and environmental factors such as exposure to certain chemicals or preserved foods.
Nasopharyngeal cancer treatment includes radiation therapy, chemotherapy, targeted therapy, and immunotherapy, based on the cancer’s stage and progression. In April 2025, the U.S. Food and Drug Administration approved penpulimab-kcqx combined with platinum-based chemotherapy for first-line treatment of adults with recurrent or metastatic non-keratinizing nasopharyngeal carcinoma, marking a significant advancement in the therapeutic pipeline.
According to the International Agency for Research on Cancer, an estimated 120,416 new cases of nasopharyngeal carcinoma (NPC) were reported globally in 2022, accounting for 0.6% of all cancer cases. The disease resulted in approximately 73,476 deaths, with significantly higher incidence and mortality in males. As per Cancer Research UK, survival rates vary by stage. Localized NPC shows over 80% five-year survival, regional 70%, and distant around 50%, based on SEER data.
This section of the report covers the analysis of nasopharyngeal cancer drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
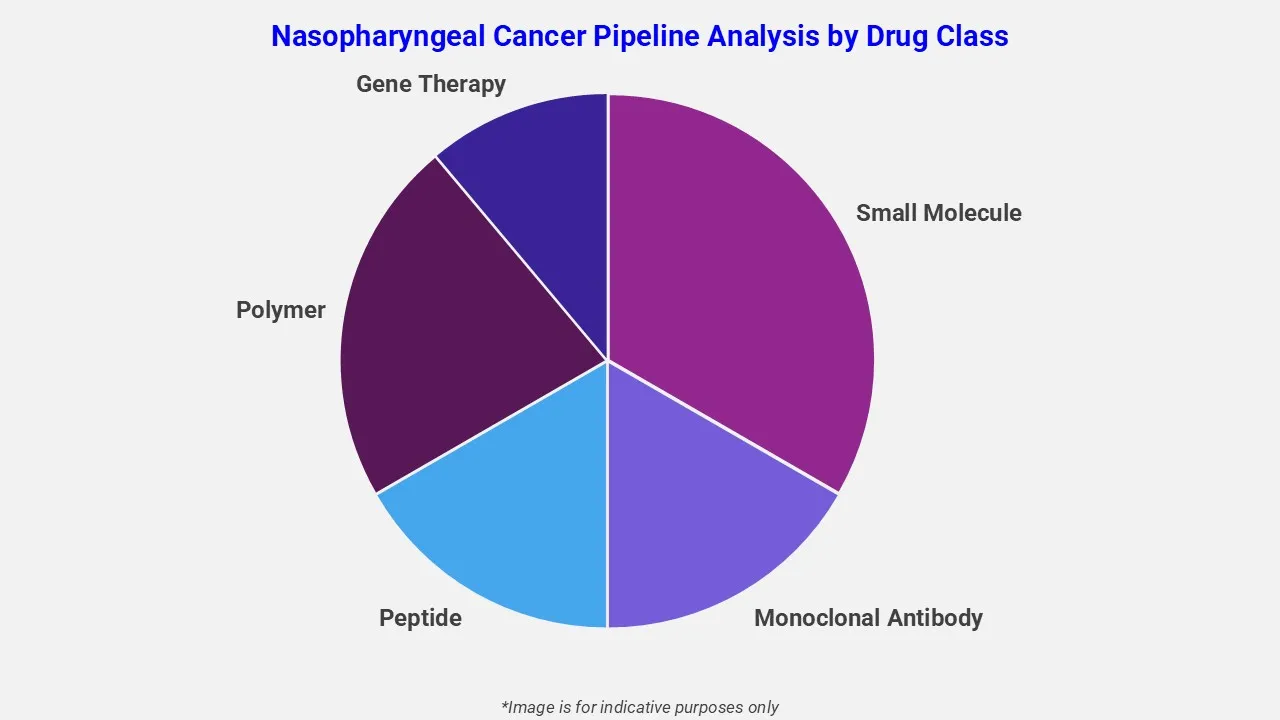
By Drug Class
The nasopharyngeal cancer pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration.
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II, with 51%, covers a major share of the total nasopharyngeal cancer clinical trials, indicating a strong focus on mid-stage development. Phase III follows with 34%, showing promising advancements toward late-stage trials. Phase I accounts for 11%. These robust developments positively influence future treatment options and market growth.
The drug molecule categories covered under the nasopharyngeal cancer pipeline analysis include small molecule, monoclonal antibody, peptide, polymer, and gene therapy. The nasopharyngeal cancer report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for nasopharyngeal cancer. Monoclonal antibody therapies are also emerging as effective treatment options for nasopharyngeal cancer. For instance, LOQTORZI (toripalimab-tpzi), an anti-PD-1 monoclonal antibody, has been approved in combination with cisplatin and gemcitabine as a first-line treatment. Additionally, it is approved as a monotherapy for recurrent or metastatic cases, enhancing immune-mediated tumor response and improving patient outcomes.
The EMR report for the nasopharyngeal cancer pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed nasopharyngeal cancer therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in nasopharyngeal cancer clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for nasopharyngeal cancer. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of nasopharyngeal cancer drug candidates.
YL201 is a B7-H3-targeting antibody-drug conjugate (ADC) is under evaluation in a Phase III trial, sponsored by MediLink Therapeutics (Suzhou) Co., Ltd. The study is comparing YL201 to investigator’s choice chemotherapy in patients with recurrent or metastatic nasopharyngeal carcinoma who have failed prior PD-(L)1 therapy and at least two chemotherapy lines. The trial aims to assess whether YL201 is improving overall survival and objective response rate while evaluating safety, pharmacokinetics, and immunogenicity. The drug features a tumor microenvironment-activable linker and a novel topoisomerase I inhibitor, designed to enhance tumor-specific cytotoxicity.
HLX43 is a novel anti-PD-L1 antibody-drug conjugate (ADC) developed by Shanghai Henlius Biotech. The ongoing Phase II study aims to evaluate the efficacy, safety, and tolerability of HLX43 in patients with recurrent or metastatic nasopharyngeal carcinoma (NPC) who have failed or are intolerant to second-line therapy. HLX43 is combining immune checkpoint inhibition with targeted cytotoxicity to deliver a potent anti-tumor response through dual mechanisms.
APG-5918 is a orally available Embryonic Ectoderm Development (EED) inhibitor, developed by Ascentage Pharma Group Inc. The Phase 1 trial is currently examining its safety, pharmacokinetics, and efficacy in patients with advanced solid tumors, including nasopharyngeal carcinoma. The study aims to determine the maximum tolerated dose and assess APG-5918’s antitumor activity. The drug is showing strong potential across multiple cancer models.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Nasopharyngeal Cancer Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for nasopharyngeal cancer. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into nasopharyngeal cancer collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share