
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Platinum-resistant relapsed ovarian cancer (PRROC) refers to ovarian cancer that recurs or progresses within six months of completing platinum-based chemotherapy. According to Yanan Ren et al., 2025, ovarian cancer accounts for about 325,000 new diagnoses globally each year, with roughly 235,000 deaths, underscoring its severity. At present, treatment options include non-platinum single-agent chemotherapies, anti-angiogenic agents, and targeted therapies, though response rates remain low. According to the platinum-resistant relapsed ovarian cancer pipeline analysis by Expert Market Research, the drug development landscape is expanding, with various pipeline candidates under evaluation. These developments, along with increased research investment, suggest that more effective and personalized PRROC therapies could emerge in the coming years.
Major companies involved in the platinum-resistant relapsed ovarian cancer pipeline analysis include Alphamab Biopharmaceuticals Co., Ltd., Precigen, Inc., and others.
Leading drugs currently in the pipeline include JSKN003, IN10018, PRGN-3005 UltraCAR-T Cells, and others.
The pipeline is witnessing strong growth, driven by the rising clinical success of targeted therapies, accelerated regulatory designations, and growing investment in combination regimens aimed at improving treatment durability and response rates.
The Platinum Resistant Relapsed Ovarian Cancer Pipeline Analysis Report by Expert Market Research gives comprehensive insights into platinum resistant relapsed ovarian cancer therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for platinum resistant relapsed ovarian cancer. The platinum resistant relapsed ovarian cancer report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The platinum resistant relapsed ovarian cancer pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with platinum resistant relapsed ovarian cancer treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to platinum resistant relapsed ovarian cancer.
Read more about this report - Request a Free Sample
Platinum-resistant relapsed ovarian cancer is a challenging form of epithelial ovarian, fallopian tube, or primary peritoneal cancer that returns despite prior treatment with platinum-based chemotherapy. This occurs when cancer cells develop mechanisms to evade the effects of platinum drugs, leading to disease recurrence. Patients face a poor prognosis and limit therapeutic options, making effective management strategies critical for improving outcomes and prolonging survival in this aggressive cancer type.
Platinum-resistant relapsed ovarian cancer is typically treated using targeted therapies, anti-angiogenic agents, combination chemotherapies, or novel immunotherapies designed to control tumor growth and extend progression-free survival. In March 2024, Suvemcitug for injection, a next-generation recombinant humanized anti-VEGF monoclonal antibody, entered the clinical pipeline for this indication. The drug selectively binds to vascular endothelial growth factor (VEGF), inhibiting tumor angiogenesis, and has demonstrated encouraging efficacy and safety in both preclinical and phase III clinical trials.
Ovarian cancer continues to be a significant global health challenge, with high mortality rates among women worldwide. Early detection remains critical for improving survival outcomes. According to Yanan Ren et al., 2025, ovarian cancer ranks fourth in female cancer mortality, with approximately 325,000 new cases and 235,000 deaths globally, accounting for 4.3% of female cancer fatalities. As per the American Cancer Society, the United States is expected to see 20,890 new diagnoses and 12,730 deaths in 2025. In the United Kingdom, over 7,000 women are diagnosed annually, with more than 4,000 deaths, as reported by Target Ovarian Cancer. In India, Abhilash Patra et al., 2024, report an incidence of 6.7%, ranking ovarian cancer among the top five female cancers. These epidemiological trends underscore the urgent need for advanced therapies and continued research in platinum-resistant relapsed ovarian cancer.
This section of the report covers the analysis of platinum resistant relapsed ovarian cancer drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
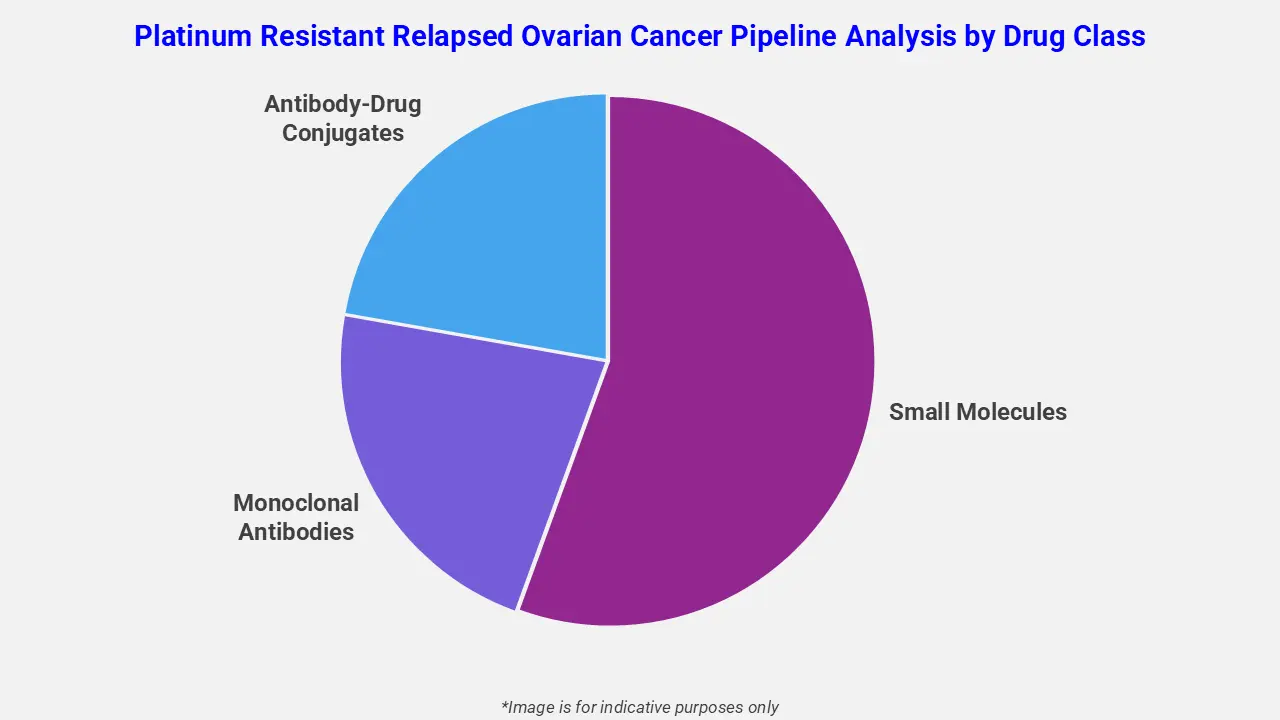
By Drug Class
The platinum resistant relapsed ovarian cancer pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration.
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II with 46%, covers a major share of the total platinum resistant relapsed ovarian cancer clinical trials. It is followed by phase I with 28%, and phase III with 23%. The strong presence of phase II and phase I candidates highlights a robust development pipeline, indicating promising future treatment options and potential market growth for this challenging oncology segment.
The drug molecule categories covered under the platinum resistant relapsed ovarian cancer pipeline analysis include small molecules, monoclonal antibodies, and antibody-drug conjugates. The platinum resistant relapsed ovarian cancer report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for platinum resistant relapsed ovarian cancer. Immune checkpoint inhibitors are emerging as a promising drug class in the platinum-resistant relapsed ovarian cancer pipeline. For instance, in May 2025, KEYTRUDA® (pembrolizumab), an anti-programmed death receptor-1 therapy, was evaluated in combination with paclitaxel, with or without bevacizumab, in the Phase 3 KEYNOTE-B96 trial. The study demonstrated significant improvements in progression-free survival and overall survival, particularly in patients with PD-L1–expressing tumors.
The EMR report for the platinum resistant relapsed ovarian cancer pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed platinum resistant relapsed ovarian cancer therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in platinum resistant relapsed ovarian cancer clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for platinum resistant relapsed ovarian cancer. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of platinum resistant relapsed ovarian cancer drug candidates.
JSKN003 is emerging as a next-generation anti-HER2 biparatopic antibody-drug conjugate sponsored by Jiangsu Alphamab Biopharmaceuticals Co., Ltd. The Phase III study aims to examine its efficacy and safety in platinum-resistant, relapsed epithelial ovarian cancer, with study completion estimated for December 30, 2027. The drug binds to HER2-expressing tumor cells and releases topoisomerase I inhibitors through endocytosis, enabling potent targeted cell killing. JSKN003 is also demonstrating improved serum stability, a stronger bystander effect, and a broader therapeutic window compared to conventional ADCs.
IN10018 is a highly selective, orally administered small molecule inhibitor targeting focal adhesion kinase, showing promising synergy with chemotherapies and immunotherapies. Developed by InxMed (Shanghai) Co., Ltd., this Phase II multicenter, randomized, double-blind study is evaluating IN10018 in combination with Pegylated Liposomal Doxorubicin (PLD) versus placebo plus PLD in patients with platinum-resistant recurrent ovarian cancer, including fallopian tube and primary peritoneal cancers. The study is enrolling approximately 168 subjects and aims to examine the safety, efficacy, and potential clinical benefit of IN10018, with an estimated completion date of December 24, 2026.
PRGN-3005 UltraCAR-T, sponsored by Precigen, Inc., is undergoing a Phase I/Ib study to evaluate its safety and determine the recommended dose in patients with advanced, recurrent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer. This investigational therapy is an autologous CAR-T cell treatment engineered to target tumor-specific proteins, co-express membrane-bound interleukin-15, and include a kill switch for precise control. The study is examining how these modified immune cells can effectively attack cancer cells while assessing tolerability and potential anti-tumor activity.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Platinum Resistant Relapsed Ovarian Cancer Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for platinum resistant relapsed ovarian cancer. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into platinum resistant relapsed ovarian cancer collaborations, regulatory environments, and potential growth opportunities.
Ovarian Cancer Drug Pipeline Analysis




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share