
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Retinal vein occlusion (RVO) is a retinal vascular disorder caused by the blockage of veins that drain blood from the retina, often leading to vision loss. According to Bingcai Jiang et al. (2025), the global prevalence of RVO is about 0.77%, equal to roughly 28 million people worldwide, imposing a substantial societal burden. Current therapies center on anti‑VEGF injections, corticosteroids, and focal laser procedures to manage macular edema and prevent further vision deterioration. According to the retinal vein occlusion pipeline analysis by Expert Market Research, robust pipeline activity involves numerous candidates across clinical stages targeting improved efficacy and longer‑acting treatments, reflecting focused innovation to address unmet needs and projected growth in RVO therapeutics in the coming years.
Major companies involved in the retinal vein occlusion pipeline analysis include Annexin Pharmaceuticals AB, MediBeacon, and others.
Leading drugs currently in the pipeline include Vamikibart, ANXV, MB-102, and others.
The pipeline is expanding with advanced anti-VEGF therapies, sustained-release formulations, and combination treatments, driven by increasing RVO prevalence and ongoing clinical trials targeting improved efficacy and patient compliance.
The Retinal Vein Occlusion Pipeline Analysis Report by Expert Market Research gives comprehensive insights into retinal vein occlusion therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for retinal vein occlusion. The retinal vein occlusion report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The retinal vein occlusion pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with retinal vein occlusion treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to retinal vein occlusion.
Read more about this report - Request a Free Sample
Retinal vein occlusion (RVO) is a common retinal vascular disorder that occurs when a vein in the retina becomes blocked, leading to blood buildup, restricted circulation, and increased ocular pressure. This blockage can cause sudden blurry vision, vision loss, and complications such as macular edema, driven by elevated vascular endothelial growth factor (VEGF) levels.
Retinal vein occlusion treatments include anti-VEGF injections, corticosteroids, laser therapy, and surgical interventions to reduce macular edema, improve vision, and prevent further retinal damage. In November 2025, EYLEA HD® (aflibercept) Injection 8 mg was approved by the FDA for treating macular edema following Retinal Vein Occlusion. The therapy enables up to every 8-week dosing after initial monthly injections, providing flexible, personalized treatment that reduces injection frequency while effectively managing vision loss and macular swelling in patients with RVO.
The pipeline is gaining momentum due to the increasing global disease burden. According to Bingcai Jiang et al., 2025, RVO affects approximately 0.77% of the global population, equivalent to 28.06 million people, posing a significant societal impact. As per Xin-yu Zhao et al., 2024, the prevalence among individuals aged 30 to 89 years mirrors this estimate. Regional studies, such as Muhammad Kamran Khalid et al., 2024, indicate a 4.18% prevalence among green laser-treated patients in Dera Ismail Khan Division, highlighting the need for targeted therapeutic interventions. Ongoing pipeline developments aim to address unmet clinical needs.
This section of the report covers the analysis of retinal vein occlusion drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
By Drug Class
The retinal vein occlusion pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II, at 29%, covers a major share of the total retinal vein occlusion clinical trials, reflecting strong clinical validation and advanced efficacy testing. Early phase I holds 21%, highlighting promising early-stage innovations and novel mechanisms. Phase III also represents 21%, emphasizing pivotal trials and regulatory readiness. Phase I accounts for 14%, showing exploratory safety studies, while Phase IV is 14%, indicating post-marketing monitoring and real-world evidence generation. This distribution supports steady pipeline growth and potential expansion of treatment options.
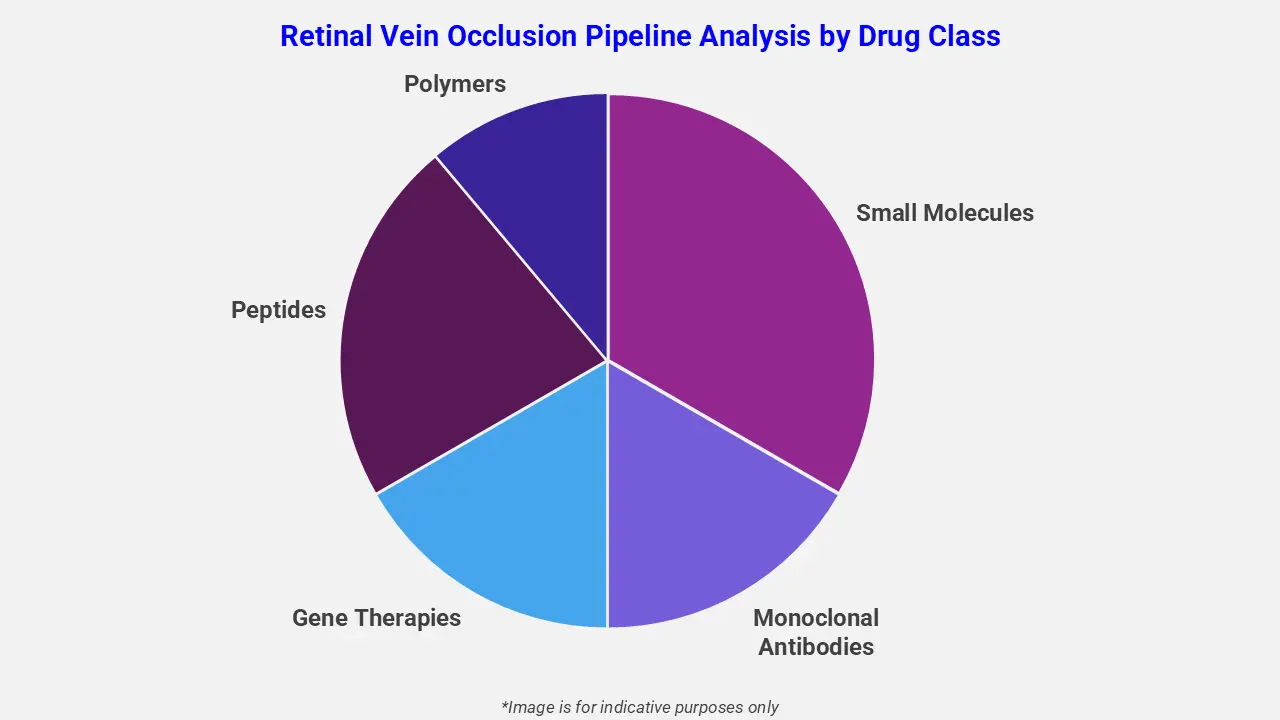
The drug molecule categories covered under the retinal vein occlusion pipeline analysis include small molecules, monoclonal antibodies, gene therapies, peptides, and polymers. The retinal vein occlusion report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for retinal vein occlusion. Anti-vascular endothelial growth factor (Anti-VEGF) therapies are actively involved in the market to address vision loss in patients. For example, Lupin Limited’s biosimilar ranibizumab, Ranluspec™, is under evaluation following a positive CHMP opinion. It works by binding to VEGF-A, inhibiting abnormal blood vessel growth, and reducing macular edema, thereby improving retinal structure and visual acuity in affected patients.
The EMR report for the retinal vein occlusion pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed retinal vein occlusion therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in retinal vein occlusion clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for retinal vein occlusion. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of retinal vein occlusion drug candidates.
Vamikibart, developed by Roche, is an investigational monoclonal antibody administered via intravitreal (IVT) injection, targeting interleukin-6 (IL-6) to reduce inflammation in retinal diseases. While it is primarily being studied in uveitic macular edema (UME), its mechanism of modulating inflammatory pathways positions it as a potential treatment for retinal vein occlusion (RVO)-related macular edema, where inflammation contributes to vision loss. In ongoing phase III MEERKAT and SANDCAT trials, the studies are examining efficacy in improving best corrected visual acuity (BCVA) and reducing central subfield thickness (CST). Vamikibart offers a non-steroid, locally injectable option for managing retinal vascular inflammation.
ANXV, a recombinant human Annexin A5 protein, is a first-in-class biologic with potentially disease-modifying mechanisms of action, being investigated by Annexin Pharmaceuticals AB in a Phase 2 clinical trial for patients with non-proliferative diabetic retinopathy or recent-onset retinal vein occlusion. Administered as a 30-minute intravenous infusion daily for five days, ANXV targets vascular and inflammatory pathways in the retina. The study is evaluating safety, tolerability, and proof-of-concept efficacy, including vision improvement, retinal imaging outcomes, and reduction in rescue medication use, comparing dose levels of 1 mg, 4 mg, and 6 mg over a four-month follow-up with comprehensive ocular and systemic assessments.
MB-102, sponsored by MediBeacon, is an investigational fluorescent tracer designed for intravenous administration to enhance retinal vascular imaging. This early Phase 1 study is evaluating the feasibility, safety, and image quality of MB-102 in ocular angiography compared to the standard fluorescein sodium. The drug works by fluorescing under light and is cleared exclusively via the kidney’s glomerular filtration, allowing precise real-time vascular imaging. The study examines both healthy participants and those with retinal conditions, capturing high-resolution fundus images to assess diagnostic utility, tolerability, and potential adverse events following MB-102 administration.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Retinal Vein Occlusion Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for retinal vein occlusion. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into retinal vein occlusion collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share