
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Seasonal influenza remains a significant global health concern, characterized by annual outbreaks that vary in intensity and viral strain circulation. According to CDC data from the 2024–25 influenza season, clinical laboratories tested 3,978,954 respiratory specimens, of which 489,579 (12.3%) were positive for influenza viruses. Influenza A accounted for 434,985 cases (88.8%), while 54,594 cases (11.2%) were identified as influenza B. These findings highlight the persistent burden of seasonal influenza and the need for continuous surveillance, updated vaccines, and improved antiviral strategies. According to the seasonal influenza pipeline analysis by Expert Market Research, influenza therapeutics and vaccines are expected to expand steadily in the coming years, driven by ongoing research efforts, evolving viral patterns, and the growing need for enhanced public health preparedness.
Major companies involved in the seasonal influenza pipeline analysis include GlaxoSmithKline, Sanofi, and others.
Leading biologicals currently in the pipeline include VRC-FLUMOS0116-00-VP, Inactivated influenza vaccine, and others.
The growth of seasonal influenza research and therapeutics is driven by recurring annual outbreaks, viral strain mutations, expanding high-risk populations, and the global demand for more effective and longer-lasting vaccines.
The Seasonal Influenza Pipeline Analysis Report by Expert Market Research gives comprehensive insights into seasonal influenza therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for Seasonal Influenza. The seasonal influenza report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The seasonal influenza pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with seasonal influenza treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to seasonal influenza.
Read more about this report - Request a Free Sample
Seasonal influenza is an acute, contagious respiratory illness caused by influenza viruses that circulate each year globally. It spreads easily through droplets and leads to symptoms such as fever, cough, sore throat, muscle pain, and fatigue. The virus undergoes frequent mutations, creating new strains that can reduce natural immunity and increase susceptibility to infection. Seasonal influenza poses a significant health risk, particularly for young children, older adults, pregnant women, and individuals with weakened immune systems, often resulting in complications such as pneumonia and hospitalization.
Seasonal influenza treatments aim to reduce symptom severity, shorten illness duration, and prevent severe outcomes. Approved options include antiviral medications that target viral replication, as well as annually updated vaccines designed to match circulating strains. In the United States, Fluzone High-Dose Quadrivalent is an FDA-approved vaccine specifically formulated to enhance immune protection in adults aged 65 years and older, offering stronger antibody responses compared to standard-dose vaccines. This advancement represents an important step in improving seasonal influenza prevention and strengthening public health preparedness.
The epidemiological burden of seasonal influenza continues to grow as annual viral circulation intensifies worldwide. According to CDC data from the 2024–25 influenza season, clinical laboratories tested 3,978,954 respiratory specimens, with 489,579 (12.3%) confirmed positive cases. Influenza A dominated the season, accounting for 434,985 infections (88.8%), while 54,594 cases (11.2%) were attributed to influenza B. These figures highlight the persistent and widespread impact of seasonal influenza across populations. The high infection rates and shifting strain patterns emphasize the ongoing need for improved surveillance systems, enhanced vaccination strategies, and continued innovation in influenza prevention and treatment.
This section of the report covers the analysis of seasonal influenza drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
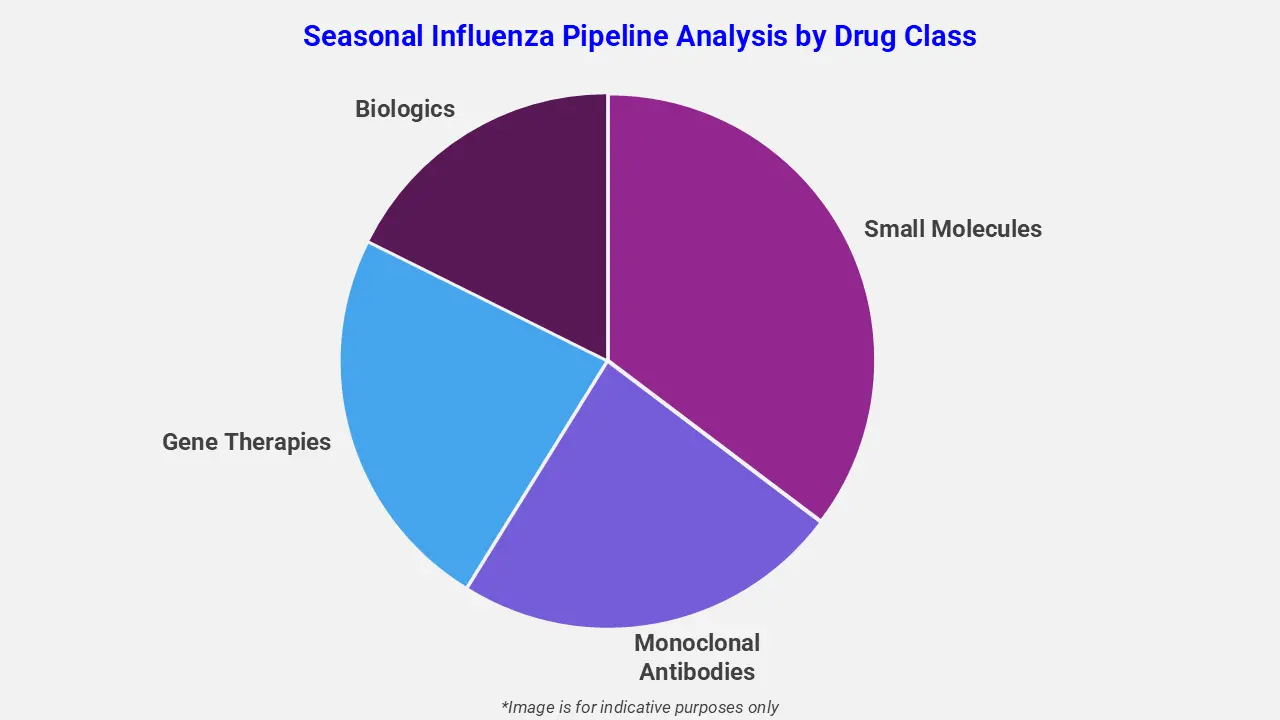
By Drug Class
The seasonal influenza pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase I, with 45%, covers a major share of the total seasonal influenza clinical trials. It is followed by the phase II at 36%, and other phases. Strong representation across all phases indicates a robust pipeline, fostering innovation and potential new therapies that can enhance treatment options and market growth.
The drug molecule categories covered under the seasonal influenza pipeline analysis include small molecules, monoclonal bodies, gene therapies, and biologics. The seasonal influenza report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for Seasonal Influenza. For instance, Flu mRNA, an investigational biologic vaccine, is currently being evaluated in Phase 2 studies to assess its ability to induce strong and broad immune responses against circulating influenza strains. This vaccine contains synthetic messenger RNA molecules that encode selected influenza antigens, enabling the body to produce viral proteins that trigger a targeted immune reaction. As a biologic based on mRNA technology, Flu mRNA offers the potential for rapid adaptation to viral mutations, enhanced immunogenicity, and a favorable safety and tolerability profile consistent with established mRNA vaccine platforms.
The EMR report for the seasonal influenza pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed seasonal influenza therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in Seasonal Influenza clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for Seasonal Influenza. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of seasonal influenza drug candidates.
The inactivated influenza vaccine, evaluated by The University of Hong Kong, is being studied in a Phase 4 clinical trial assessing twice-annual vaccination in older adults. This study investigates both the Northern Hemisphere and Southern Hemisphere formulations to determine their immunogenicity, seasonal protection, and overall effectiveness in an aging population. A placebo group is included to enable comparative assessment of immune response and vaccine performance across dosing schedules. The trial is designed to generate evidence on whether biannual vaccination enhances immunity in regions with year-round viral circulation. The study is active but not recruiting and is expected to be completed between September 2025 and September 2026.
The Flu mRNA vaccine formulations, developed by GlaxoSmithKline, are being evaluated in a Phase 2 clinical study in adults 18 years of age and older. These investigational mRNA-based influenza vaccines, tested across multiple formulations, are designed to enhance immune responses and improve protection against circulating influenza strains. The study compares the immunogenicity and safety of the Flu mRNA candidates with several comparator vaccines to assess their potential advantages in efficacy and tolerability. This interventional trial aims to generate robust clinical evidence supporting next-generation influenza vaccine development. The study is currently recruiting and is expected to be completed by September 2026.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Seasonal Influenza Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for Seasonal Influenza. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into seasonal influenza collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share