
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
The Asia Pacific drug development market was valued at USD 50.29 Billion in 2025, driven by the rapid growth of the pharmaceutical sector and the rising innovations in drug development processes in the region. The market is expected to grow at a CAGR of 7.10% during the forecast period of 2026-2035, with the values likely to reach USD 99.86 Billion by 2035.
Base Year
Historical Period
Forecast Period
The increasing prevalence of chronic diseases in the Asia Pacific region is fuelling the market demand for drug development.
The rising investments in technologies that speed up the drug development process are likely to boost the market size in the forecast period.
The growing adoption of digital health technologies like wearable devices, mobile health applications, and telemedicine in the drug development processes is a significant market trend.
Compound Annual Growth Rate
7.1%
Value in USD Billion
2026-2035
*this image is indicative*
Drug development involves the process of bringing a novel drug to the market. It comprises activities such as preclinical research, the discovery of lead compounds, clinical trials, and marketing approval from the regulatory bodies. The drug development phase accounts for around two-thirds of the overall R&D costs, with the cost rising sharply as the drug candidate moves closer to the later phases of clinical development.
The drug development market in the Asia Pacific is driven by the rising burden of chronic diseases such as cardiovascular conditions, diabetes, and cancer, coupled with the growing elderly population. Further, the rapid growth of the biotechnological and pharmaceutical sectors is propelling the market demand. Moreover, the increasing investments in healthcare infrastructure, technological innovations in drug development processes, and the favorable regulatory environment are poised to impact the market dynamics in the Asia Pacific region.
Increasing Prevalence of Chronic Diseases to Affect the Market Landscape Significantly
According to the Longitudinal Ageing Survey in India (LASI), around 21% of the aging population is affected by at least one chronic disease in India. This prevalence rate is 29% in the urban areas compared to 17% in the rural areas. Hypertension and diabetes comprise 68% of all chronic diseases among older adults whereas cardiovascular conditions affect 37% of people aged 75 years and above. The increasing prevalence of chronic diseases in India and other countries in the Asia Pacific region is fuelling the market demand for drug development.
The market is witnessing several trends and developments to improve the current scenario in Asia Pacific. Some of the notable trends are as follows:
“Drug Development Market Report and Forecast 2026-2035” offers a detailed analysis of the market based on the following segments:
Market Breakup by Product Type
Market Breakup by Application
Market Breakup by Delivery Type
Market Breakup by End User
Market Breakup by Region
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Market Segmentation Based on Application is Anticipated to Witness Substantial Growth
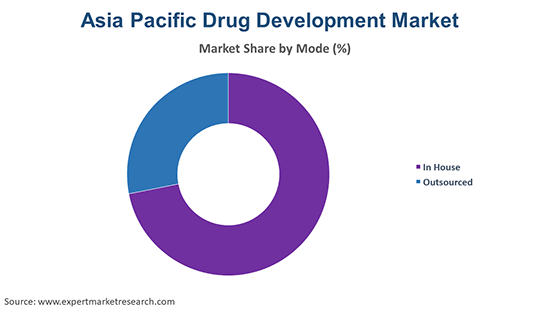
Based on the application, the market is segmented into drug development and drug discovery, among other applications. In the drug development segment, the clinical trial component involving multiple phases is resource-intensive and requires substantial investment. There is a growing trend of outsourcing clinical trials and regulatory submissions to contract research organizations (CROs), which is likely to propel innovation in the segment. Further, the rising development of biologics and biosimilars is anticipated to boost the growth of the drug development segment.
The market segmentation by region includes Japan, India, ASEAN, Australia, and others. Japan represents a major market for drug development in the Asia Pacific region owing to the high burden of chronic diseases, a substantial aging population, and a well-established pharmaceutical industry. Further, the presence of a favorable regulatory environment and government support for pharmaceutical research are likely to propel market growth in the region.
The key features of the market report comprise patent analysis, grants analysis, funding and investment analysis, partnership and collaborations analysis, and strategic initiatives by the leading key players. The major companies in the market are as follows:
Takeda Pharmaceutical Company Limited, headquartered in Tokyo, Japan, is one of the key players in the market. The company is known for its substantial investment in research and development to bring new therapies to the market. In April 2024 , Takeda Pharma inked a partnership agreement with the Japanese Foundation for Cancer Research (JFCR) to accelerate the development of new potential cancer therapies.
Novartis AG, headquartered in Basel, Switzerland, has a prominent presence in the market and focuses on the development and manufacturing of prescription and generic drugs across various therapeutic areas. In July 2024, Novartis AG’s subsidiary Novartis Pharma AG entered into a strategic collaboration with Dren Bio, Inc., a clinical-stage biopharmaceutical company specializing in developing antibody therapeutics for cancer and other diseases, with the aim to advance innovative therapies for cancer.
Global biopharmaceutical company Pfizer is known for its robust digital technologies and quantitative strategies to improve the likelihood of success for its drug candidates and accelerate clinical development. The company leverages real-world data, integrates artificial intelligence, and automates repetitive processes to bring novel drugs swiftly to the market.
Swiss healthcare company F. Hoffmann-La Roche plays a significant role in the growth of the market. The company boasts a strong portfolio of cancer therapies, including targeted biologics and immunotherapies. Roche actively engages in collaborations with research institutions and biotech companies to expedite the development of new treatments.
*Please note that this is only a partial list; the complete list of key players is available in the full report. Additionally, the list of key players can be customized to better suit your needs.*
Other key players in the market include GSK plc, AstraZeneca PLC, Sanofi S.A., Johnson & Johnson Services, Inc., Eli Lilly and Company, Viatris Inc., and Daiichi Sankyo Company Limited.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
| REPORT FEATURES | DETAILS |
| Base Year | 2025 |
| Historical Period | 2019-2025 |
| Forecast Period | 2026-2035 |
| Scope of the Report |
Historical and Forecast Trends, Industry Drivers and Constraints, Historical and Forecast Market Analysis by Segment:
|
| Breakup by Product Type |
|
| Breakup by Application |
|
| Breakup by Delivery Type |
|
| Breakup by End User |
|
| Breakup by Region |
|
| Market Dynamics |
|
| Supplier Landscape |
|
| Companies Covered |
|
Datasheet
One User
USD 2,699
USD 2,429
tax inclusive*
Single User License
One User
USD 4,299
USD 3,869
tax inclusive*
Five User License
Five User
USD 5,799
USD 4,949
tax inclusive*
Corporate License
Unlimited Users
USD 6,999
USD 5,949
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share