
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
The global autogenous vaccines market was valued at USD 144.30 Million in 2025. The market is anticipated to grow at a CAGR of 5.60% during the forecast period of 2026-2035, with the values likely to reach USD 248.83 Million by 2035. The rising demand for farm-specific, rapid-response vaccines is poised to drive the market.
Base Year
Historical Period
Forecast Period
The market trends include rising expansions by key companies. For instance, Ceva’s manufacturing expansion in May 2024 strengthens global supply chains and improves access to vaccines.
USDA’s June 2024 approval for live aMPV vaccine imports supports broader use of autogenous solutions.
Rising collaborations between pharma and diagnostics are expected to accelerate tailored vaccine production and improve farm-specific disease management.
Compound Annual Growth Rate
5.6%
Value in USD Million
2026-2035
*this image is indicative*
The market addresses the growing demand for farm-specific solutions against localized or emerging pathogens, particularly in the livestock and poultry sectors. These custom-made vaccines, derived from farm-isolated organisms, offer targeted protection when commercial options fall short. Rising concerns over antibiotic resistance, evolving disease strains, and regulatory support for tailored veterinary interventions are driving market growth. Autogenous vaccines are increasingly utilized alongside conventional vaccines to enhance biosecurity and safeguard animal health, particularly in poultry, swine, and aquaculture industries. The market is anticipated to grow at a CAGR of 5.60% during the forecast period of 2026-2035.
Growing Focus on Customized Vaccines Driving Market Expansion
The rising incidence of avian respiratory diseases and the increasing demand for targeted, site-specific animal health solutions are propelling the market growth. For instance, in August 2024, Merck Animal Health launched an experimental autogenous vaccine specifically for avian metapneumovirus (aMPV) Type B, derived from U.S. isolates. This initiative strengthens farm-specific disease control strategies and showcases industry efforts to provide rapid, tailored solutions. Such developments are expected to significantly accelerate the market growth during the forecast period by addressing unmet poultry health needs.
Innovative Disease Control Strategies Enhancing Autogenous Vaccines Market Demand
The rising regulatory acceptance of autogenous vaccines and the growing need for rapid outbreak response in poultry health are key market drivers. For instance, in September 2024, Ceva Animal Health commenced production of an experimental autogenous aMPV vaccine formulated from U.S.-origin isolates, aiming to address emerging localized strains effectively. This strategic move is anticipated to boost the market by expanding access to customized, farm-specific solutions, further encouraging adoption in regions where commercial vaccine options may not offer adequate protection.
Major market trends include the expansion of manufacturing capacities, regulatory advancements, and rising demand for tailored solutions.
Expanding Manufacturing Capacity to Strengthen Access and Support Growing Market Demand
The growing emphasis on expanding manufacturing capacities to meet the rising demand for customized animal health solutions is significantly shaping the market. For instance, in May 2024, Ceva Animal Health announced its investment in a new 7,000 m² vaccine manufacturing facility to expand its global production capabilities. This development highlights the industry's commitment to scaling up vaccine availability to address localized disease outbreaks. Such capacity expansions are poised to strengthen market growth by ensuring reliable supply chains, improved distribution reach, and faster response to emerging health threats across various livestock and poultry sectors during the forecast period.
Regulatory Advancements to Enable Autogenous Vaccines Market Growth
Regulatory approvals and government support aimed at enhancing disease prevention strategies in the livestock and poultry industries are contributing to the growing development of the market. For instance, in June 2024, the USDA approved the first import of a live avian metapneumovirus (aMPV) vaccine for use in U.S. poultry farms. This milestone demonstrates how evolving regulatory frameworks are fostering broader adoption of both autogenous and complementary live vaccine solutions. These advancements are expected to drive further innovation, enhance farm-specific disease management options, and positively influence the global autogenous vaccine market development in the coming years.
Rising Demand for Site-Specific Solutions Bolstering Autogenous Vaccines Market Value
The increasing demand for farm-specific, strain-targeted vaccines is emerging as a critical factor boosting the market value. For instance, in recent years, growing disease outbreaks caused by localized and evolving pathogens, particularly in poultry and swine, have highlighted the limitations of commercial vaccines. This trend is pushing veterinary pharmaceutical companies to enhance their focus on bespoke vaccine solutions, driving growth by offering precise, faster, and more reliable protection tailored to specific farm needs.
Collaborative Industry Efforts to Increase Autogenous Vaccines Market Demand
Strategic collaborations between pharmaceutical companies and diagnostic laboratories are playing a vital role in advancing the global autogenous vaccine market. For instance, partnerships are increasingly being established to streamline pathogen identification, isolate collection, and rapid vaccine production processes. These collaborative efforts ensure the timely delivery of customized vaccines to farms, enhancing disease control efficiency. As a result, such industry synergies are expected to support continuous innovation, improve operational efficiencies, and strengthen the development prospects of the market globally.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Livestock to Lead the Market Segmentation by Animal Type
The livestock segment is expected to continue holding the largest market share due to the widespread use of autogenous vaccines in cattle, pigs, and sheep. These animals are highly vulnerable to localized and evolving diseases, making farm-specific vaccines essential for effective disease management. Livestock also represents a significant portion of global animal production, increasing the need for reliable preventive measures. This consistent demand for customized vaccines to protect herd health strongly supports the growth and dominance of the livestock segment in the market.
North America is expected to maintain the largest market share due to its advanced regulatory environment, growing preference for farm-specific vaccines, and early adoption of innovations like the USDA-approved live aMPV vaccine in 2024. Historically, the region accounted for over 26% of the market revenue, highlighting its leadership. In contrast, Europe is also witnessing steady growth, supported by stringent animal health regulations and increased demand for customized disease management solutions. Both regions are likely to dominate, while other regions contribute moderately through gradual adoption.
The key features of the market report comprise patent analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:
Founded in 1994, Newport Laboratories is headquartered in Worthington, Minnesota, and operates as part of Vaxxinova/Boehringer Ingelheim. Specializing in custom vaccines for swine and cattle, the company also offers diagnostic testing services. Their autogenous vaccines are tailored to specific herd pathogens. Newport supports rapid on-farm responses, making it a trusted partner for veterinarians and producers seeking fast, farm-specific immunization strategies.
Established in 1954 as part of Eli Lilly, Elanco is now headquartered in Greenfield/Indianapolis, Indiana, and listed on the NYSE (ELAN). Boasting a global portfolio, Elanco offers pharmaceutical products and vaccines for both livestock and companion animals. Following its 2019 separation from Lilly, it acquired Bayer’s animal health assets in 2020, expanding its vaccine capabilities and strengthening its global market position.
Founded in 2017 and based in Worthington, Minnesota, Cambridge Technologies focuses on USDA-approved autogenous vaccines. Their expertise includes molecular diagnostics, pathogen isolation, and in-house vaccine production, particularly for poultry and livestock. In April 2024, they partnered with Merck Animal Health to distribute custom poultry vaccines, underscoring their innovative edge in tailored biologic solutions.
Operating for over 30 years from Davis, California, Hygieia develops both conventional and autogenous veterinary vaccines alongside diagnostic tools. Serving livestock, poultry, aquaculture, and companion animals, they emphasize precise, site-specific pathogen protection. Hygieia also collaborates with local governments to provide subsidized vaccines in rural areas, enhancing access to customized veterinary health interventions.
*Please note that this is only a partial list; the complete list of key players is available in the full report. Additionally, the list of key players can be customized to better suit your needs.*
Other players in the market include AniCon Labor GmbH, Boehringer Ingelheim International GmbH, Ceva Biovac, Phibro Animal Health Corporation, Huvepharma, Inc., and Ace Laboratory Services.
Autogenous Vaccines Market Report and Forecast 2026-2035” offers a detailed analysis of the market based on the following segments:
Market Breakup by Strain Type
Market Breakup by Technology
Market Breakup by Animal Type
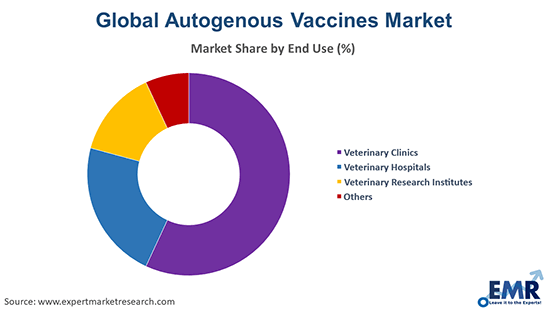
Market Breakup by End User
Market Breakup by Region




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
| REPORT FEATURES | DETAILS |
| Base Year | 2025 |
| Historical Period | 2019-2025 |
| Forecast Period | 2026-2035 |
| Scope of the Report |
Historical and Forecast Trends, Industry Drivers and Constraints, Historical and Forecast Market Analysis by Segment:
|
| Breakup by Strain Type |
|
| Breakup by Technology |
|
| Breakup by Animal Type |
|
| Breakup by End User |
|
| Breakup by Region |
|
| Market Dynamics |
|
| Supplier Landscape |
|
| Companies Covered |
|
| Report Price and Purchase Option | Explore our purchase options that are best suited to your resources and industry needs. |
| Delivery Format | Delivered as an attached PDF and Excel through email, with an option of receiving an editable PPT, according to the purchase option. |
Datasheet
One User
USD 3,299
USD 2,969
tax inclusive*
Single User License
One User
USD 5,499
USD 4,949
tax inclusive*
Five User License
Five User
USD 6,999
USD 5,949
tax inclusive*
Corporate License
Unlimited Users
USD 8,199
USD 6,969
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share