
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
The North America in‐vitro diagnostics market was valued at USD 53.40 Billion in 2025 and is expected to grow at a CAGR of 4.70%, reaching USD 84.53 Billion by 2035. The market growth is driven by rising prevalence of chronic and infectious diseases, greater demand for early detection tests, and expanding point‐of‐care diagnostics.
Base Year
Historical Period
Forecast Period
Compound Annual Growth Rate
4.7%
Value in USD Billion
2026-2035
*this image is indicative*
In-vitro diagnostics can help identify early warning signs and individual risk factors, generating new openings for prevention and early intervention. Generally, in-vitro diagnostics (IVD) tests are considered medical gadgets. These may include reagents, techniques, instruments, or a combination thereof employed in-vitro for the investigation of samples such as blood, urine or tissue to obtain a diagnosis from assays in a controlled environment outside a living organism. Several leading companies offer in-vitro diagnostic (IVD) solutions that deliver rapid, sensitive results while minimizing cost and complexity. Modern in-vitro diagnostics (IVDs) enable better diagnosis for prevention and management of infectious and chronic diseases. These factors are expected to drive market growth.

Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
A majority of in-vitro diagnostics market is concentrated in developed nations, key being USA, Europe and Japan; the in-vitro diagnostics market, overall, is led by major players including Roche Diagnostics, Abbott Diagnostics, Siemens, Johnson & Johnson Medical Devices and Diagnostics, Beckman Coulter and BioMerieux. Roche Diagnostics is a key player in in-vitro diagnostics and tissue-based cancer diagnostics. In 2020, 23 billion tests were carried out across the world using the company’s diagnostics products. Companies like Roche are engaged in understanding how diseases differ at the molecular level, and developing novel tests and drugs that prevent, diagnose and treat such illnesses. Diagnosis is an important and complex activity; accurate diagnosis is vital to ensure correct treatment. Roche Diagnostics Canada offers a distinct and wide product portfolio, with advanced solutions to physicians, hospitals, laboratories, patients, and researchers, in the domains of immunology, clinical chemistry, molecular biology, point-of-care, pathology and life science.
The domain of point-of-care (POC) diagnostics offers the likelihood of providing rapid diagnostic results in non-laboratory environments. With increasingly connected consumer electronic devices, patients and consumers are progressively changing their interaction with POC medical technologies in conjunction with mobile devices; consumers are looking to manage and take control of their own health. POC diagnostic and monitoring devices are likely to increase in popularity in various environments, including home, hospital, clinic, and in developed as well as developing nations. Point of care (POC) diagnostics comprises a significant portion of the total in-vitro diagnostics market in the USA, with the US dominating the general POC market; rising popularity of POC diagnostics is expected to stimulate the growth of the North America in-vitro diagnostics market.
Major companies are looking to broaden their offerings and come together to enhance capabilities. For example, in 2023, Medix Biochemica acquired all shares of Bioresrouce Technology to widen its base matrix abilities and further reinforce its local presence in the United States market. Through the partnership, Medix Biochemica would provide its customers an extensive offering of raw materials for IVD quality control products. The organisation and operations of BRT would form a significant portion of Medix Biochemica’s IVD Biomaterials Business Unit, which also comprised manufacturing of native antigens, enzymes, proteins and biologicals in St. Louis, Missouri. Such developments are likely to boost the North America in-vitro diagnostics market.
In 2023, ALine, Inc. and Nectar Product Development announced a strategic partnership to advance point-of-care IVD medical products. The partnership sought to get the microfluidic consumable design to interface robustly with the instrument while fulfilling all instrument design and performance requirements.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
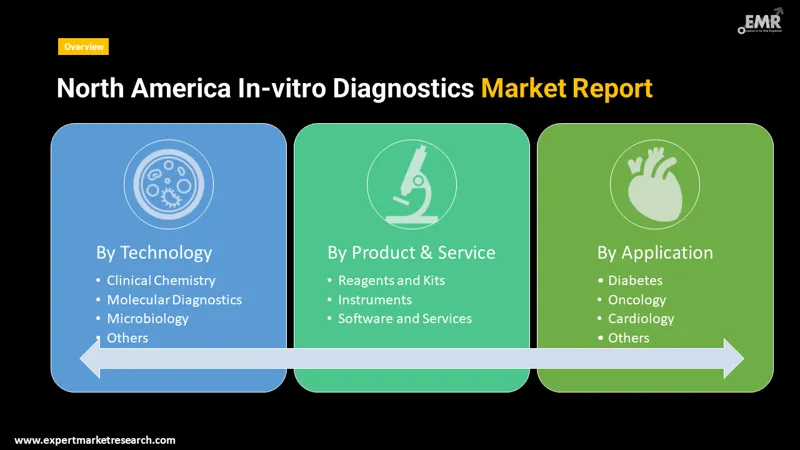
North America In-vitro Diagnostics Market Report and Forecast 2026-2035 offers a detailed analysis of the market based on the following segments:
By product and service, the market is segmented into
By technology, the market is divided into
By application, the market is divided into
By Region, the market is classified into
The report presents a detailed analysis of the following key players in the North America in-vitro diagnostics market, looking into their capacity, and latest developments like capacity expansions, plant turnarounds, and mergers and acquisitions:
The EMR report gives an in-depth insight into the industry by providing a SWOT analysis as well as an analysis of Porter’s Five Forces model.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
The market is projected to grow at a CAGR of 4.70% between 2026 and 2035.
The major drivers of the North America in-vitro diagnostics market include the technological advancements, rising levels of income, rising standards of living, newly insured patients, and modernised healthcare facilities.
The rising popularity of point of care diagnostics and growing demand for quality medical care are the key industry trends propelling the market's growth.
The major countries in the industry are United States and Canada.
The various products and services in the market are reagents and kits, instruments, software and services.
Based on technology, the market is segmented into immunoassay/immunochemistry, clinical chemistry, molecular diagnostics, microbiology, haematology, blood glucose self-monitoring, coagulation and haemostasis, and urinalysis, among others.
The major applications of the market are infectious diseases, diabetes, cardiology, oncology, autoimmune diseases, and nephrology, among others.
The major players in the industry are Becton, Dickinson and Company, Beckman Coulter, Inc., Bio-Rad Laboratories, Inc., Surmodics IVD, Inc., and Illumina, Inc., among others.
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
| REPORT FEATURES | DETAILS |
| Base Year | 2025 |
| Historical Period | 2019-2025 |
| Forecast Period | 2026-2035 |
| Scope of the Report |
Historical and Forecast Trends, Industry Drivers and Constraints, Historical and Forecast Market Analysis by Segment:
|
| Breakup by Product and Service |
|
| Breakup by Technology |
|
| Breakup by Application |
|
| Breakup by Region |
|
| Market Dynamics |
|
| Supplier Landscape |
|
| Companies Covered |
|
Datasheet
One User
USD 2,699
USD 2,429
tax inclusive*
Single User License
One User
USD 4,299
USD 3,869
tax inclusive*
Five User License
Five User
USD 5,799
USD 4,949
tax inclusive*
Corporate License
Unlimited Users
USD 6,999
USD 5,949
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share