
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
The spinal muscular atrophy treatment market was valued at USD 6.62 Billion in 2025, driven by the high prevalence of the disorder, spreading awareness, and technological advancements in diagnostics and treatment across the 8 major markets. The market is anticipated to grow at a CAGR of 14.60% during the forecast period of 2026-2035 to achieve a value of USD 25.86 Billion by 2035.
Base Year
Historical Period
Forecast Period
The market is set for significant growth, driven by advancements in gene therapies, increasing newborn screening initiatives, and rising awareness of early intervention benefits.
Expanding government support, improved healthcare infrastructure, and ongoing clinical research into novel therapeutics are expected to boost the adoption of targeted spinal muscular atrophy treatments.
The introduction of innovative RNA-based and small-molecule therapies, along with increasing patient access to advanced treatments, is likely to enhance market expansion and improve long-term disease management outcomes.
Compound Annual Growth Rate
14.6%
Value in USD Billion
2026-2035
*this image is indicative*
Spinal muscular atrophy (SMA) treatment focuses on improving motor function, slowing disease progression, and enhancing quality of life. Therapies include gene replacement therapy, such as onasemnogene abeparvovec, which targets the genetic cause of SMA. Antisense oligonucleotide drugs, like nusinersen, enhance SMN protein production, improving nerve function. Risdiplam, an oral therapy, increases SMN protein levels systemically. Supportive care, including physiotherapy, respiratory support, and nutritional management, is crucial for patient well-being. Advances in gene therapy and personalised medicine continue to transform SMA treatment, offering improved prognosis and mobility for affected individuals.

Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Increasing Adoption of Innovative Therapies to Drive Market Growth
The rising prevalence of spinal muscular atrophy and the increasing demand for advanced therapies are significant market drivers. Additionally, ongoing innovations in muscle-targeted therapies and enhanced treatment regimens are expected to fuel market growth. For instance, in October 2024, Scholar Rock announced promising results from the Phase 3 SAPPHIRE clinical trial evaluating apitegromab, an investigational muscle-targeted treatment for SMA. The therapy demonstrated a clinically significant improvement in motor function, offering a potential treatment option for SMA patients currently on standard care therapies. These positive results are poised to enhance treatment outcomes and drive market expansion in the forecast period.
Regulatory Advancements to Meet Rising Spinal Muscular Atrophy Treatment Market Demands
The growing emphasis on early diagnosis and treatment of SMA alongside evolving therapeutic solutions is propelling the treatment market. For instance, In January 2025, Biogen Inc. revealed that both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) accepted applications for a higher dose regimen of nusinersen, which will offer a more rapid loading dose and extended maintenance dosing. This improved dosing regimen promises to optimise treatment efficacy, addressing unmet needs in SMA care and further expanding the therapeutic market. This development is anticipated to boost market growth in the coming years.
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
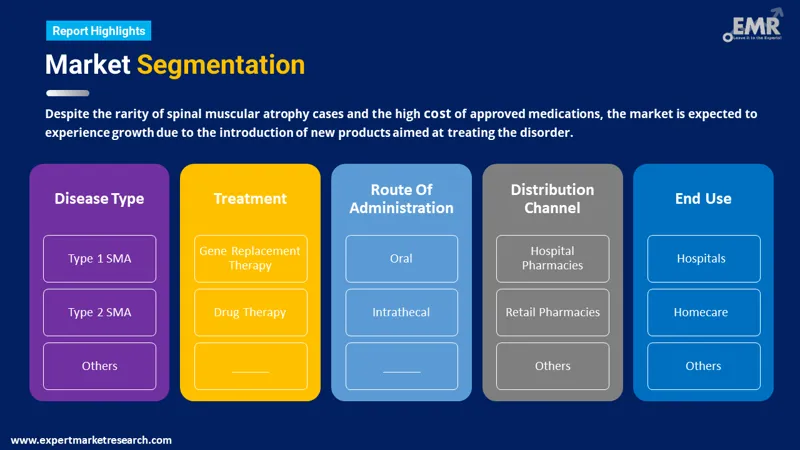
Spinal Muscular Atrophy Treatment Market Report and Forecast 2026-2035 offers a detailed analysis of the market based on the following segments
Market Breakup by Type
Market Breakup by Procedure
Market Breakup by Route of Administration
Market Breakup by End User
Market Breakup by Region
Type 1 to Lead the Segmentation by Type
Type 1 spinal muscular atrophy (SMA) is the most severe and prevalent form, accounting for a significant portion of the spinal muscular atrophy patient population. Due to the urgent need for effective treatments, this segment is expected to hold the largest market share in the forecast period. Advances in gene therapies, such as Zolgensma, which specifically targets Type 1 SMA, are driving market growth. As these therapies offer life-changing benefits, the demand for treatments for Type 1 SMA will continue to increase. The success of Type 1 treatment options sets the tone for future growth in the treatment market.
Gene Replacement Therapy to Dominate Spinal Muscular Atrophy Treatment Market Segmentation by Procedure
Gene replacement therapy is poised to emerge as a leading procedure segment in the treatment market. The success of therapies like Zolgensma has made gene replacement a groundbreaking treatment option. By addressing the root cause of SMA, gene therapy provides long-term, transformative results compared to traditional drug therapies. With ongoing advancements in gene editing techniques and regulatory support, this segment is poised to dominate. The growing acceptance of gene therapies, along with their ability to offer permanent cures, is expected to propel this segment forward, driving significant market expansion in the coming years.
Parenteral Route to Hold a Major Spinal Muscular Atrophy Treatment Market Value by Route of Administration
The parenteral route of administration, particularly intravenous (IV) injection or infusion, is amongst the commonly used methods for delivering spinal muscular atrophy treatments. With gene therapies like Zolgensma requiring intravenous infusion, this route of administration is likely to hold the largest market share in the forecast period. Parenteral delivery ensures optimal bioavailability, especially for biologic therapies that need to bypass the digestive system for effective absorption. The trend towards IV-based gene therapies, with their ability to provide targeted and long-lasting effects, will continue to drive the market for parenteral SMA treatments.
Hospitals to Lead the Spinal Muscular Atrophy Treatment Market by End User
Hospitals are expected to lead the end-user segment for spinal muscular atrophy treatments due to the critical nature of SMA care and the specialised treatments required. These institutions are equipped with the necessary infrastructure, medical professionals, and facilities to administer complex therapies such as gene replacement and drug therapies. As SMA treatments become more advanced and require specialised monitoring and administration, hospitals are expected to maintain dominance in the market. With an increasing number of healthcare institutions adopting cutting-edge SMA therapies, hospitals are poised to drive the highest demand and expansion of SMA treatment solutions.
The United States is likely to hold the largest market share in the market. This is primarily due to its well-established healthcare infrastructure, high awareness of SMA, and significant investment in biotechnology research. Additionally, the FDA's approval of innovative therapies such as gene therapy and RNA-based treatments has created a conducive environment for market growth. Europe, particularly Germany and the United Kingdom is also witnessing strong market expansion due to favourable reimbursement policies and increasing access to advanced therapies. Japan, with its robust healthcare system, is expected to show growth, while emerging markets like India are anticipated to contribute steadily with rising awareness and healthcare improvements.
The key features of the market report comprise patent analysis, clinical trial analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:
Founded in 1978 and headquartered in Cambridge, Massachusetts, USA, Biogen Inc. is a global biotechnology company known for its leadership in neuroscience. Specialising in treatments for neurological diseases, Biogen's portfolio includes therapies for spinal muscular atrophy (SMA), multiple sclerosis, and Alzheimer's disease. Their innovations in gene therapies and biologics have positioned Biogen as a key player in the biopharmaceutical industry, particularly in rare and complex neurological disorders. The company is also involved in research aimed at advancing treatments in neurology and neurodegenerative diseases.
Established in 1996 and headquartered in Basel, Switzerland, Novartis AG is a leading global healthcare company focusing on patented prescription medicines. Their portfolio covers innovative pharmaceuticals, generics, and biosimilars, particularly in areas like oncology, immunology, ophthalmology, and neuroscience. Novartis has made significant contributions to the treatment of spinal muscular atrophy (SMA) and other rare diseases. The company continues to invest heavily in research and development, aiming to enhance the lives of patients globally through cutting-edge therapies and pioneering scientific advancements.
Founded in 1896 and based in Basel, Switzerland, F. Hoffmann-La Roche Ltd is a multinational healthcare leader in pharmaceuticals and diagnostics. Known for its pioneering work in oncology, immunology, and neurology, Roche’s portfolio includes treatments for rare diseases, autoimmune disorders, and neurological conditions like SMA. The company’s commitment to personalised healthcare has made it a key player in the global biotechnology space. Roche continues to drive innovation in both pharmaceutical therapies and diagnostic tools, improving outcomes for patients worldwide.
Founded in 1989 and headquartered in Carlsbad, California, USA, Ionis Pharmaceuticals Inc. is a biotechnology company focused on RNA-targeted therapies. Ionis is a pioneer in the development of antisense oligonucleotide therapies and is known for its role in advancing treatments for rare genetic disorders, including spinal muscular atrophy (SMA). The company’s innovative pipeline includes therapies for a range of conditions, from neurological to cardiovascular diseases. Ionis collaborates with global pharmaceutical companies to expand its reach and impact in treating complex diseases.
*Please note that this is only a partial list; the complete list of key players is available in the full report. Additionally, the list of key players can be customized to better suit your needs.*
Other key players in the market include Cytokinetics Inc., PTC Therapeutics, Catalyst Pharmaceuticals, Astellas Pharma Inc., Pfizer Inc., and NMD Pharma A/S.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
| REPORT FEATURES | DETAILS |
| Base Year | 2025 |
| Historical Period | 2019-2025 |
| Forecast Period | 2026-2035 |
| Scope of the Report |
Historical and Forecast Trends, Industry Drivers and Constraints, Historical and Forecast Market Analysis by Segment:
|
| Breakup by Type |
|
| Breakup by Procedure |
|
| Breakup by Route of Administration |
|
| Breakup by End User |
|
| Breakup by Region |
|
| Market Dynamics |
|
| Supplier Landscape |
|
| Companies Covered |
|
Single User License
One User
USD 5,499
USD 4,949
tax inclusive*
Datasheet
One User
USD 3,299
USD 2,969
tax inclusive*
Five User License
Five User
USD 6,999
USD 5,949
tax inclusive*
Corporate License
Unlimited Users
USD 8,199
USD 6,969
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share