
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Cutaneous Melanoma is an aggressive form of skin cancer that originates from melanocytes, the cells responsible for producing skin pigment, and is known for its high potential to spread to other parts of the body. According to Jiaxiang Xu et al., 2025, the global incidence of malignant cutaneous melanoma is rising steadily, increasing by approximately 2.6% annually, highlighting the growing disease burden worldwide. The drug pipeline for cutaneous melanoma is increasingly focused on advanced immunotherapies, targeted treatments such as BRAF and MEK inhibitors, and innovative combination strategies aimed at improving survival outcomes. Rising UV exposure, growing awareness, and unmet therapeutic needs in advanced stages are key factors driving research efforts. According to the cutaneous melanoma pipeline analysis by Expert Market Research, continued investments in research and development are expected to support strong growth and innovation in the coming years.
Major companies involved in the cutaneous melanoma pipeline analysis include Immatics US, Inc., Krystal Biotech, Inc., and others.
Leading drugs currently in the pipeline include IMA203, LNS8801, KB707, and others.
The strong late-stage immunotherapies, expanding targeted mutation-specific treatments, and rising combination regimens are accelerating clinical progress, strengthening approvals potential, and driving sustained pipeline expansion over coming years.
The Cutaneous Melanoma Pipeline Analysis Report by Expert Market Research gives comprehensive insights into cutaneous melanoma therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for cutaneous melanoma. The cutaneous melanoma report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The cutaneous melanoma pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with cutaneous melanoma treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to cutaneous melanoma.
Read more about this report - Request a Free Sample
Cutaneous melanoma is a malignant skin cancer that develops from melanocytes, the cells responsible for pigment production. It commonly occurs due to prolonged ultraviolet radiation exposure from sunlight or tanning devices, leading to DNA damage and uncontrolled cell growth. Genetic predisposition and weakened immune function also increase disease risk.
Cutaneous melanoma treatment typically includes surgical excision, immunotherapy, targeted therapy, chemotherapy, and radiation therapy, depending on disease stage, tumor mutation profile, and patient health status. In January 2023, the United States Food and Drug Administration granted Orphan Drug Designation to LNS8801 for metastatic Cutaneous Melanoma. The oral G Protein-Coupled Estrogen Receptor agonist demonstrated safety, target engagement, and promising antitumor activity in ongoing Phase I/II clinical trials.
The pipeline reflects the rising disease burden that continues to drive therapeutic innovation worldwide, as epidemiology indicates a steady increase in incidence across regions and age groups. According to Jiaxiang Xu et al., 2025, the global incidence of malignant cutaneous melanoma is growing by approximately 2.6% annually, while as per the Melanoma Research Foundation, nearly 187,000 new melanoma cases and about 7,990 deaths were expected in the United States in 2023, with higher growth among younger populations. According to Alice Indini et al., 2024, incidence increases significantly from one per million in children aged ≤14 years to 71 per million in adolescents and young adults aged 15–39 years in Europe, underscoring an expanding patient population fueling drug pipeline growth.
This section of the report covers the analysis of cutaneous melanoma drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
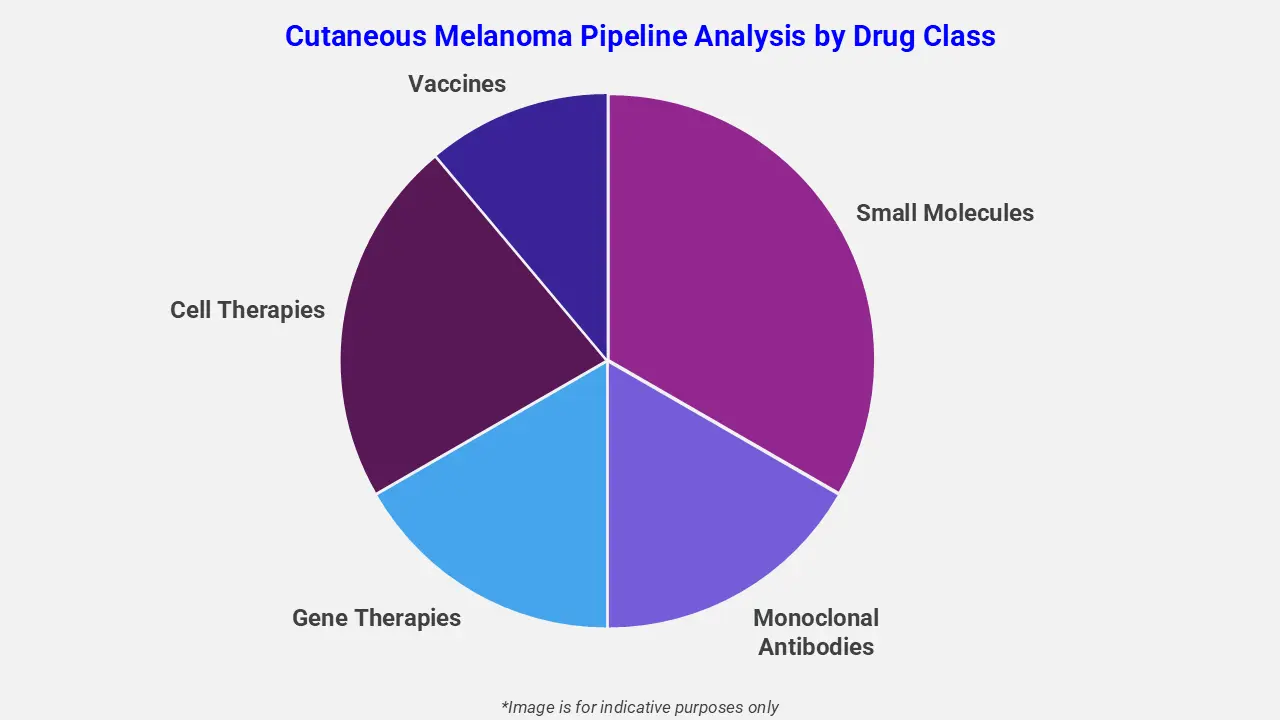
By Drug Class
The cutaneous melanoma pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II, with 47%, covers a major share of the total cutaneous melanoma clinical trials, driven by advanced clinical trials, promising efficacy, and targeted therapy innovations. Phase I holds 41%, supported by strong early-stage research, novel drug candidates, and robust safety evaluations. Phase III accounts for 9%, reflecting late-stage validation and potential regulatory approvals.
The drug molecule categories covered under the cutaneous melanoma pipeline analysis include small molecules, monoclonal antibodies, gene therapies, cell therapies, and vaccines. The cutaneous melanoma report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for cutaneous melanoma. ImmTAC-based immunotherapies are gaining attention in the cutaneous melanoma pipeline as novel treatment options. For example, KIMMTRAK (tebentafusp-tebn), a bispecific protein targeting gp100, is being evaluated as monotherapy and in combination with pembrolizumab in a Phase 3 TEBE-AM trial for previously treated advanced cutaneous melanoma. It redirects T cells to selectively kill tumor cells, offering potential survival benefits.
The EMR report for the cutaneous melanoma pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed cutaneous melanoma therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in cutaneous melanoma clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for cutaneous melanoma. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of cutaneous melanoma drug candidates.
IMA203 is a PRAME-directed TCR T-cell therapy being sponsored by Immatics US, Inc. and is currently being evaluated in a Phase 3 clinical trial for previously treated, unresectable, or metastatic cutaneous melanoma. This study examines the efficacy, safety, and tolerability of IMA203 compared with the investigator’s choice of standard treatments. The drug is an autologous, genetically engineered T-cell product designed to recognize PRAME-derived peptides presented by HLA-A*02:01 on tumor cells, triggering a targeted immune response. IMA203 is being administered via intravenous infusion following lymphodepletion, with supportive low-dose IL-2 therapy.
LNS8801 is an oral small-molecule agonist of the G-protein coupled estrogen receptor (GPER) being developed by Linnaeus Therapeutics, Inc. for treatment-refractory cutaneous melanoma. The ongoing Phase 2/3 study is examining the safety, tolerability, and antitumor activity of LNS8801 as monotherapy and in combination with pembrolizumab. The objective is to evaluate progression-free survival and immune response enhancement in genetically selected patients. LNS8801 works by promoting melanocytic differentiation, inhibiting tumor proliferation, reducing c-Myc expression, and improving immune recognition of melanoma cells.
KB707 is a genetically modified herpes simplex virus type 1–based oncolytic immunotherapy being sponsored by Krystal Biotech, Inc. for the treatment of unresectable or metastatic cutaneous melanoma. The ongoing Phase 1/2 study is examining the safety, tolerability, immunologic activity, and preliminary efficacy of KB707, both as a monotherapy and in combination with immune checkpoint inhibitors such as Opdualag and Keytruda. The drug is working by locally delivering cytokines IL-12 and IL-2 into the tumor microenvironment to stimulate anti-tumor immune responses and is being administered through intratumoral injections at regular intervals.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Cutaneous Melanoma Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for cutaneous melanoma. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into cutaneous melanoma collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share