
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Dystrophic epidermolysis bullosa (DEB) is a rare, inherited skin disorder characterized by extremely fragile skin that blisters and tears easily due to mutations in the COL7A1 gene. According to Divya Gupta et al., 2023, the incidence of DEB is approximately 40 cases per million live births, with a prevalence near 21 per million individuals. According to the dystrophic epidermolysis bullosa pipeline analysis by Expert Market Research, the therapeutic landscape is evolving with gene therapies, protein replacement treatments, and novel biologics in clinical development. The growing focus on wound healing, gene correction, and long-term disease modification is expected to drive significant market growth in the coming years.
Major companies involved in the dystrophic epidermolysis bullosa pipeline analysis include Krystal Biotech, Inc., Eliksa Therapeutics, Inc., and others.
Leading drugs currently in the pipeline include KB803, FCX-007, ELK-003, and others.
The pipeline shows robust growth driven by gene and cell therapy advancements, increasing clinical trials, and rising investment in targeted molecular therapies, highlighting strong innovation and expanding treatment options in the coming years.
The Dystrophic Epidermolysis Bullosa Pipeline Analysis Report by Expert Market Research gives comprehensive insights into dystrophic epidermolysis bullosa therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for dystrophic epidermolysis bullosa. The dystrophic epidermolysis bullosa report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The dystrophic epidermolysis bullosa pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with dystrophic epidermolysis bullosa treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to dystrophic epidermolysis bullosa.
Read more about this report - Request a Free Sample
Dystrophic epidermolysis bullosa (DEB) is a rare inherited skin disorder caused by mutations in the COL7A1 gene, resulting in defective type VII collagen. The condition weakens anchoring fibrils between the epidermis and dermis, leading to extremely fragile skin, recurrent blistering, chronic wounds, nail deformities, and scarring from birth.
Dystrophic epidermolysis bullosa treatment primarily focuses on wound care, pain control, infection prevention, and supportive management to reduce complications. Emerging therapies increasingly target the underlying genetic cause. In May 2023, Vyjuvek, a herpes-simplex virus type 1 vector-based topical gene therapy, advanced the DEB drug pipeline by delivering functional COL7A1 genes, enabling anchoring fibril formation and improved wound healing outcomes.
The pipeline reflects increasing clinical and research focus on this rare genetic condition. Epidemiological evidence highlights a measurable disease burden. According to Divya Gupta et al., 2023, the incidence of epidermolysis bullosa is estimated at 41.3 cases per million live births. As per Harshita Prabhakaran et al., 2023, the incidence in the United States is around 20 per million births. Additionally, Maria L. Bageta et al., 2023, reported a United Kingdom prevalence of 34.8 per million population. These figures continue to drive innovation across genes, cell, and protein-based therapeutic pipelines.
This section of the report covers the analysis of dystrophic epidermolysis bullosa drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
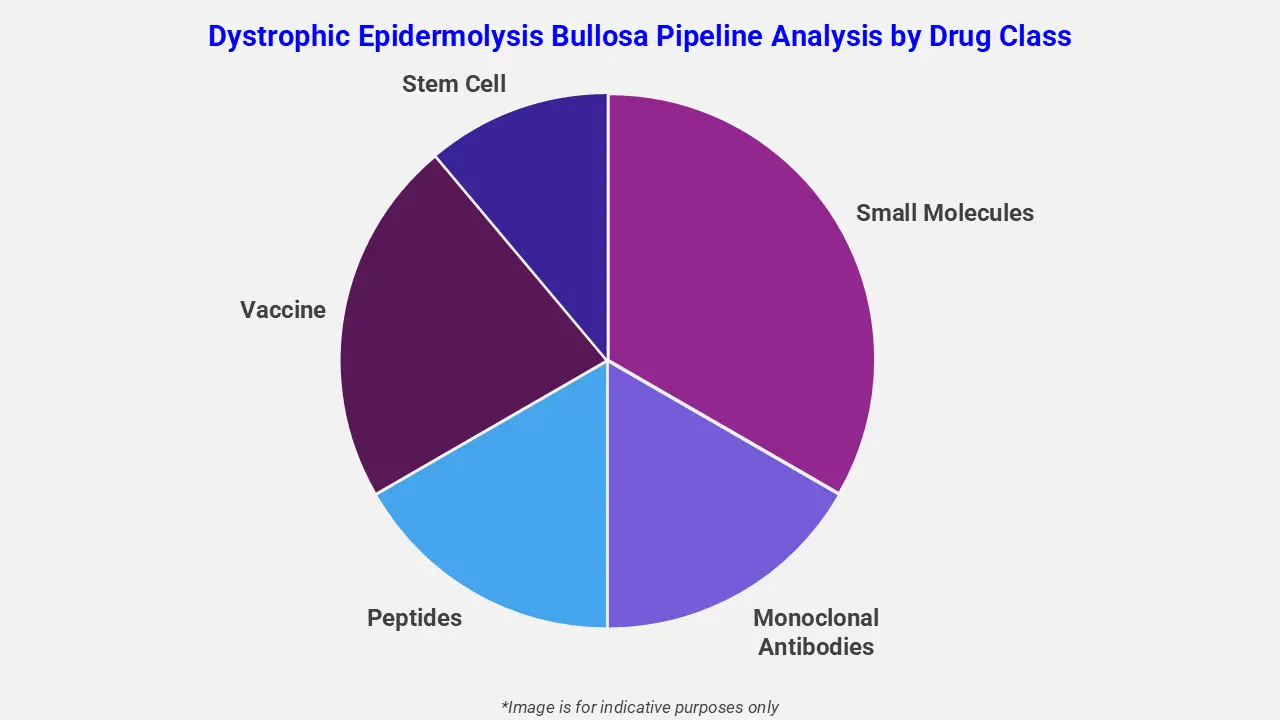
By Drug Class
The dystrophic epidermolysis bullosa pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II covers a major share of the total dystrophic epidermolysis bullosa clinical trials, with 39%, reflects strong mid-stage clinical momentum. Phase I represents 35%, followed by phase III at 22%, and other phases. This balanced phase distribution supports sustained innovation, progressive clinical validation, and long-term growth potential.
The drug molecule categories covered under the dystrophic epidermolysis bullosa pipeline analysis include small molecules, monoclonal antibodies, peptides, vaccines, and stem cells. The dystrophic epidermolysis bullosa report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for dystrophic epidermolysis bullosa. Gene-based therapies represent a rapidly advancing drug class in the dystrophic epidermolysis bullosa drug pipeline, offering disease-modifying potential. For instance, prademagene zamikeracel, an autologous gene-corrected epidermal sheet therapy, has received regulatory approval for recessive dystrophic epidermolysis bullosa. It delivers a functional COL7A1 gene, thereby promoting durable wound healing and pain reduction.
The EMR report for the dystrophic epidermolysis bullosa pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed dystrophic epidermolysis bullosa therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in dystrophic epidermolysis bullosa clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for dystrophic epidermolysis bullosa. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of dystrophic epidermolysis bullosa drug candidates.
KB803 is an innovative, re-dosable ophthalmic gene therapy developed by Krystal Biotech, Inc., engineered to deliver two copies of the COL7A1 transgene directly to the epithelial cells of the eye. This Phase 3 study is examining the safety and efficacy of KB803 in preventing and treating recurrent corneal abrasions in pediatric and adult patients with dystrophic epidermolysis bullosa (DEB). KB803 is working at the molecular level by enabling epithelial cells to produce functional type VII collagen, the protein missing in DEB, thereby addressing the root cause of the disease. Administered as weekly eye drops in the home setting by a healthcare provider, the therapy is designed to promote corneal healing and improve visual acuity. The study uses a double-blind, intra-patient crossover design comparing KB803 with matched placebo over two 12-week periods, while participants record symptom diaries and complete monthly questionnaires to monitor disease progression, treatment response, and safety outcomes.
FCX-007 is a genetically modified autologous human dermal fibroblast therapy being developed by Castle Creek Biosciences, LLC, for the treatment of recessive dystrophic epidermolysis bullosa (RDEB). This Phase 3 study examines whether intradermal injections of FCX-007, in addition to standard care, can improve wound healing compared to standard care alone. The drug works by delivering a functional version of collagen VII, which is absent in RDEB patients, directly into the skin. Subjects receive multiple treatment sessions over several months, with ongoing safety and efficacy assessments through one year, followed by long-term follow-up up to 15 years.
ELK-003 is a topical eye drop formulation derived from standardized amniotic fluid secretome, rich in extracellular matrix proteins such as collagen VII and laminin-332, which are essential for maintaining corneal integrity in dystrophic epidermolysis bullosa (DEB). Sponsored by Fundación DEBRA Chile and Niños Piel de Cristal, this Phase 1 pilot study is examining the safety and efficacy of ELK-003 when applied six times daily in patients with junctional and recessive dystrophic EB. The study is assessing ocular signs and symptoms through OCT imaging, slit lamp exams, keratography, and patient-reported outcomes, monitoring corneal abrasions, healing, and quality of life over six months.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Dystrophic Epidermolysis Bullosa Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for dystrophic epidermolysis bullosa. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into dystrophic epidermolysis bullosa collaborations, regulatory environments, and potential growth opportunities.
Recessive Dystrophic Epidermolysis Bullosa (RDEB) Pipeline Analysis Report




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share