
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Pouchitis is a common inflammatory complication in patients with ileal-pouch-anal anastomosis (IPAA), affecting patients with a cumulative incidence rising to 72% over ten years. Chronic and recurrent cases represent a substantial unmet clinical need. The pouchitis pipeline analysis by Expert Market Research highlights a diverse development landscape, including antibiotics, biologics, live biotherapeutics, and microbiome-targeted therapies, reflecting growing industry focus on precision interventions and novel mechanisms to improve long-term management and patient outcomes in pouchitis worldwide.
Major companies involved in the pouchitis pipeline analysis include Calibr, (A division of Scripps Research), McMaster University and others.
Leading drugs currently in the pipeline include CLF065, Rifaximin 550 MG Oral Tablet [XIFAXAN], and others.
Rising recognition of microbiome-driven pouch inflammation, coupled with accelerating development of novel biologics, live biotherapeutics, and microbial-restoration strategies, is uniquely driving the pouchitis pipeline. Increasing identification of antibiotic-refractory phenotypes and demand for precision, mechanism-targeted therapies further fuel innovation, attracting sustained industry investment in this highly unmet, niche gastrointestinal segment.
The Pouchitis Pipeline Analysis Report by Expert Market Research gives comprehensive insights into pouchitis therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for pouchitis. The pouchitis report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The pouchitis pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with pouchitis treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to pouchitis.
Read more about this report - Request a Free Sample
The treatment landscape for pouchitis is rapidly evolving as researchers move beyond traditional antibiotics to therapies targeting microbiome imbalance and immune-driven inflammation. Ciprofloxacin and metronidazole remain first-line for acute cases, but chronic and recurrent patients continue to face limited options. In July 2024, a new IBD biologic received regulatory approval, highlighting the potential of gut-selective integrin and cytokine-targeted therapies for pouchitis. This approval is expected to accelerate investment and development of novel antibiotics, live biotherapeutics, and immunomodulators, signaling a promising era for pouchitis management.
Pouchitis affects a substantial proportion of patients with ileal-pouch-anal anastomosis (IPAA), with approximately 37% experiencing clinical inflammation. Globally, the cumulative incidence rises rapidly, reaching 48% within two years and 72% over ten years post-surgery. Both acute and chronic forms contribute to significant morbidity, with recurrent episodes observed in a notable subset. The prevalence is consistent across adult populations undergoing IPAA for ulcerative colitis, emphasizing the persistent clinical burden. These epidemiological trends highlight the continuing need for effective preventive measures and therapeutic interventions in pouchitis management.
This section of the report covers the analysis of pouchitis drug candidates based on several segmentations, including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
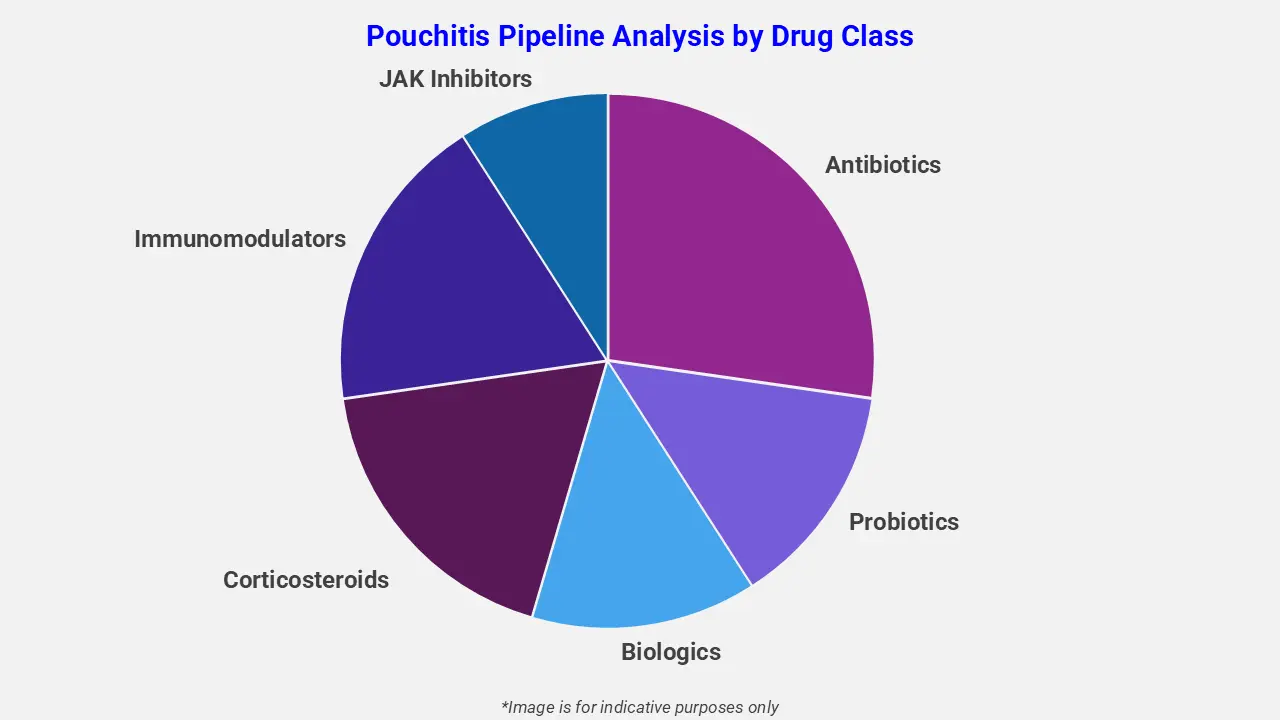
By Drug Class
The pouchitis pipeline analysis report covers 50+ drug analyses based on drug classes:
By Route of Administration
The pipeline assessment report covers 50+ drug analyses based on the route of administration.
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, early Phase 1 and Phase 1 each account for 10%, Phase 2 represents 30%, Phase 3 accounts for 20%, and Phase 4 comprises 40% of pouchitis clinical trials. Phase 4 dominates, reflecting ongoing mid-stage development and strong pipeline activity.
The drug molecule categories covered under the pouchitis pipeline analysis include antibiotics, probiotics, biologics, corticosteroids, immunomodulators, and JAK inhibitors. The pouchitis report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for pouchitis. For instance, in July 2024 the FDA granted orphan‑drug designation to epigallocatechin gallate (EGCG), a green‑tea derived molecule, for pouchitis treatment, highlighting rising interest in novel microbiome‑modulating natural compounds as part of the evolving therapeutic landscape.
The EMR report for the pouchitis pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed pouchitis therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in pouchitis clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for pouchitis. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of pouchitis drug candidates.
CLF065 is an investigational oral, gut-targeted small molecule designed to modulate inflammatory pathways relevant to chronic pouchitis. It works by reducing mucosal inflammation and restoring epithelial immune balance within the ileal pouch, aiming to improve outcomes in antibiotic-refractory disease. Its mechanism focuses on dampening pathogenic cytokine signaling while preserving mucosal integrity. CLF065 is currently being developed by Castle Creek Biosciences, a company advancing therapies for high-need gastrointestinal and inflammatory disorders.
Rifaximin 550 mg is a non-systemic oral antibiotic of the rifamycin class that targets gut bacterial overgrowth and dysbiosis, key drivers of pouchitis inflammation. Its localized activity decreases pathogenic bacterial load while supporting microbial balance in the ileal pouch. Rifaximin is frequently evaluated for chronic or recurrent pouchitis due to its favorable safety profile and limited systemic absorption. The product is developed and marketed by Salix Pharmaceuticals (Bausch Health), a major gastroenterology-focused company conducting multiple GI-related clinical programs.
Vedolizumab is a gut-selective monoclonal antibody targeting the α4β7 integrin, preventing lymphocyte migration into the intestinal mucosa. In pouchitis, it reduces immune-mediated inflammation in antibiotic-refractory or chronic cases by limiting leukocyte trafficking to the pouch. Its gut specificity makes it a preferred biologic for patients with prior IBD-associated pouch complications. Vedolizumab is developed by Takeda Pharmaceuticals, a leader in immunology and gastroenterology drug development with ongoing clinical investigations across IBD and pouchitis indications.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Pouchitis Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for pouchitis. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into pouchitis collaborations, regulatory environments, and potential growth opportunities.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share