
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
The Expert Market Research report, titled “Cefalexin Monohydrate BP Manufacturing Plant Project Report 2026 Edition: Industry Trends, Capital Investment, Price Trends, Manufacturing Process, Raw Materials Requirement, Plant Setup, Operating Cost, and Revenue Statistics,” provides an in-depth and comprehensive examination of the financial and operational aspects of establishing a cefalexin monohydrate BP plant.
The report is the result of extensive primary and secondary research, offering a detailed analysis of current market trends. It profiles key industry players, giving insights into their market strategies, production capacities, and financial performance, which are crucial for benchmarking and competitive analysis.
It delves into historical, current, and forecasted price trends, helping stakeholders understand market dynamics and price volatility. The report provides a thorough analysis of the mass balance and raw materials requirements, ensuring a clear understanding of the input-output ratios essential for efficient production. Detailed examinations of the various unit operations integral to the cefalexin monohydrate BP manufacturing process are included, highlighting process optimisation techniques and technological advancements.
The report presents a comprehensive capital cost analysis, detailing the financial investment required for setting up a cefalexin monohydrate BP plant. This includes an exhaustive breakdown of costs associated with raw materials, catchem, utilities, labour, packaging, transportation, land acquisition, construction, and machinery. Additionally, it offers an in-depth look at the operating costs, providing clarity on the recurring expenses involved in running the plant.
Projected profit margins and optimal product pricing strategies are outlined, offering guidance on maximising profitability. The report also addresses regulatory frameworks, environmental impacts, and sustainability measures pertinent to the cefalexin monohydrate BP industry.
Cefalexin monohydrate BP is a first-generation cephalosporin antibiotic, widely used for treating bacterial infections such as pharyngitis, otitis media, skin and soft tissue infections, and urinary tract infections. It disrupts bacterial cell wall synthesis by binding to penicillin-binding proteins, causing cell lysis. Developed in the 1960s by Eli Lilly and Company, it was among the first oral cephalosporins, offering a broader spectrum than penicillin and greater stability against beta-lactamase-producing bacteria. Cefalexin is well-absorbed orally, typically prescribed in doses of 250-500 mg every 6-12 hours. However, the antibiotic can cause side effects like gastrointestinal upset, rash, and rare allergic reactions.
Cefalexin monohydrate BP is a white to yellowish crystalline powder with a molecular formula of C16H17N3O4S·H2O and a molecular weight of 365.41 g/mol. It is slightly soluble in water (approximately 1.2 mg/mL at 25°C) and alcohol, but practically insoluble in acetone and chloroform. Its melting point ranges between 194–200°C, with decomposition occurring beyond this range. The compound has a pKa of approximately 4.5, reflecting its acidic functional groups. Its specific optical rotation is between +145° and +180° (1% solution in 0.1M hydrochloric acid). The cephalosporin nucleus features a beta-lactam ring, which is crucial for its bactericidal activity.
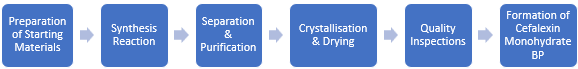
The production process of cephalexin monohydrate BP begins with the preparation of starting materials, specifically 7-Aminocephalosporanic Acid (7-ADCA) and an L-Phenylglycine ester derivative. These components are then combined in a reaction where L-Phenylglycine ester is added to an aqueous solution of 7-ADCA, with the introduction of immobilised penicillin acylase to catalyse the synthesis. Following the synthesis, the crude cephalexin powder is separated through centrifugation.
The next phase involves purification, which includes washing the crude powder with deionised water and filtering it until the liquid is clear. The pH is then adjusted using sulfuric acid to facilitate further purification, followed by a final filtration through a 0.45 µm membrane to remove any remaining impurities. After purification, crystallisation occurs by neutralising cephalexin hydrochloride with a base in aqueous methanol, resulting in the formation of cephalexin monohydrate. The slurry is cooled, and the crystallised product is filtered out. Finally, the collected cephalexin monohydrate crystals are dried to yield the final product. Throughout this process, quality control tests are conducted to ensure that the product meets the British Pharmacopoeia (BP) specifications for purity and quality.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
1. Synthesis of Cephalosporin C
The process starts with the production of a cephalosporin nucleus. The compound cephalosporin C is produced from 7-aminocephalosporanic acid (7-ACA), a key intermediate in the production of cephalosporin antibiotics.
Reaction 1:
7 - ACA + L-Valine → Cephalosporin C (with enzyme catalysis)
2. Conversion of Cephalosporin C to Cefalexin
The next step involves the modification of cephalosporin C into cefalexin by the introduction of an appropriate side chain. This is done via a chemical reaction involving nucleophilic substitution.
Reaction 2:
Cephalosporin C + Chloracetic acid → Cefalexin (reaction with base)
3. Crystallisation of Cefalexin
After the synthesis, cefalexin is purified, and the product is converted into its monohydrate form through a crystallisation process, usually involving the use of a suitable solvent like water or an alcohol.
Reaction 3:
Cefalexin + H2O → Cefalexin Monohydrate (through crystallisation)
4. Purification of Cefalexin Monohydrate
The crude cefalexin monohydrate is then purified to remove impurities and by-products through filtration and recrystallisation techniques. The final product is dried under controlled conditions to remove excess water and to obtain the monohydrate form.
Chemical Formulae:
7-Aminocephalosporanic Acid (7-ACA): C8H10N2O4S
Cefalexin: C16H17N3O4S
Cefalexin Monohydrate: C16H17N3O4S · H2O
The cephalexin monohydrate BP market is driven by its extensive applications in treating a variety of bacterial infections, including respiratory tract infections, skin infections, urinary tract infections (UTIs), and bone infections. In 2024, an estimated 3–5 million people are suffering from severe influenza globally and around 650,000 deaths attributed to this respiratory disease annually. Skin infections, often caused by bacteria such as Staphylococcus aureus, contribute to a substantial number of outpatient visits, with millions affected each year. Urinary tract infections (UTIs) are also prevalent, affecting approximately 150 million people worldwide annually, and are a leading cause of antibiotic prescriptions. Additionally, bone infections, or osteomyelitis, occur in about 2 to 5 cases per 10,000 individuals each year. The increasing prevalence of these infections, coupled with rising antibiotic resistance, has heightened the demand for effective alternatives like cephalexin, especially among patients with penicillin allergies. Government health reports indicate that cephalexin is also valuable in pediatric medicine due to its safety profile and efficacy in treating infections in children aged one year and older. Furthermore, cephalexin's role in preventing bacterial endocarditis before dental procedures underscores its significance in clinical practice. As healthcare systems continue to focus on the importance of effective infection management, the demand for cephalexin monohydrate BP is expected to grow.
This production cost analysis report by Expert Market Research scrutinises the cefalexin monohydrate BP manufacturing process, offering a comprehensive overview necessary for stakeholders considering venturing into this sector. Based on the latest economic data, the report encompasses detailed insights into the primary process flow, raw material requirements, reactions involved, utility costs, operating costs, capital investments, pricing strategies, and profit margins. This report is an indispensable resource for entrepreneurs, investors, researchers, consultants, business strategists, and all those who have any kind of stake in the cefalexin monohydrate BP industry. It equips them with essential information and strategic insights to effectively navigate the complexities of the market.
The following sections detail the comprehensive scope of the prefeasibility report for a cefalexin monohydrate BP production plant:
This prefeasibility report aims to equip potential investors and existing manufacturers with crucial insights to make informed decisions in the cefalexin monohydrate BP industry.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Basic Plan
USD 5,699
USD 4,844
Get Startedtax inclusive*
Raw Material and Product Specification, Raw material consumption, Process flow diagram
Machinery Cost, Working Capital
Utilities consumption, Operating cost, Overheads, Financing Charges, GSA , Packaging
Premium Plan
USD 6,799
USD 5,779
Get Startedtax inclusive*
Key Processing Information, Capital Investment Analysis, Conversion Cost Analysis
Raw material consumption and prices, Utilities consumption breakdown, By-Product Credit, Labour Charges Breakdown
Land and Site Cost, Equipment Cost, Auxiliary Equipment Cost, Contingency, Engineering and Consulting Charges
Enterprise Plan
USD 8,899
USD 7,564
Get Startedtax inclusive*
Key Processing Information, Capital Investment Analysis, Conversion Cost Analysis, Variable Cost Breakdown, Investing Cost Breakdown,
Breakdown of machinery cost by equipment, Auxiliary Equipment Cost, Piping, Electrical, Instrumentation
Cost of Construction, Plant Building, Site Development Charges
Land Cost, Development Charges
Dynamic Spreadsheet (Unlocked)
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*

Basic Plan
USD 5,699
USD 4,844
Key Processing Information
Raw Material and Product Specification, Raw Material Consumption, Process Flow Diagram
Capital Investment Analysis
Machinery Cost, Working Capital
Conversion Cost Analysis
Utilities Consumption, Operating Cost, Overheads, Financing Charges, GSA , Packaging

Premium Plan
USD 6,799
USD 5,779
All Contents of Basic Report
Key Processing Information, Capital Investment Analysis, Conversion Cost Analysis
Variable Cost Breakdown
Raw Material Consumption and Prices, Utilities Consumption, Breakdown By-Product Credit, Labour Charges Breakdown
Investing Cost Breakdown
Land and Site Cost, Equipment Cost, Auxiliary Equipment Cost, Contingency, Engineering and Consulting Charges

Enterprise Plan
USD 8,899
USD 7,564
Includes all Report Content
Key Processing Information, Capital Investment Analysis, Conversion Cost Analysis, Variable Cost Breakdown, Investing Cost Breakdown,
Equipment Cost Breakdown
Breakdown of Machinery Cost By Equipment, Auxiliary Equipment Cost, Piping, Electrical, Instrumentation
Land and Construction Cost Details
Land Cost, Development Charges, Cost of Construction, Plant Building, Site Development Charges
Dynamic Excel Cost Model
Dynamic Spreadsheet (Unlocked)
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share