
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
The global surgical sutures market value was USD 4.49 Billion in 2025, driven by the increasing number of surgical procedures across the globe. The market size is anticipated to grow at a CAGR of 6.00% during the forecast period of 2026-2035 to achieve a value of USD 8.04 Billion by 2035.
Base Year
Historical Period
Forecast Period
Compound Annual Growth Rate
6%
Value in USD Billion
2026-2035
*this image is indicative*
Apart from closing skin wounds, sutures have other applications also, including closing of fascia, intestinal anastomosis and enterotomy, haemostasis, surgical procedures related to the musculoskeletal system, urogenital tract surgery, ocular surgery, cardiovascular surgery, plastic surgery, neurosurgery, and others. Continual technological developments in suture material have led to increased variety of sutures available. North America, Asia and Europe are likely to be key markets.
Surgical sutures are widely used to close wounds (especially after invasive surgery) in a manner that prevents infection and bleeding. A surgical suture helps hold body tissue together after an injury or post-surgery. A suture is applied with the help of a needle and a connected thread. Sutures are available in different shapes, sizes, and materials. Such available variety is expected to drive the global surgical sutures market.
Classification of surgical sutures may be done in many ways. For example, the suture material may be grouped as absorbable suture or non-absorbable suture. Absorbable sutures are naturally absorbed by enzymes present in body tissue, and doctors need not remove them. On the other hand, non-absorbable sutures are generally removed a few days after the surgical procedure. Sometimes, depending on the type of surgery performed, non-absorbable sutures may permanently be left in the body. Absorbable sutures are grouped as Catgut Sutures (natural, monofilament absorbable suture displaying decent tensile strength; fit for use in healing tissue quickly due to the suture’s property of eventual disintegration), Polydioxanone Sutures (synthetic monofilament suture; this polydioxanone suture is used to heal various types of soft-tissue wounds, abdominal closures, and in paediatric cardiac processes), Poliglecaprone Sutures (synthetic monofilament suture, usually employed to repair soft tissues; these sutures enable aesthetic, mark-free healing), Polyglactin Sutures (synthetic braid suture, fit to be used to repair face and hand lacerations; a preferred alternative for general soft tissue approximation).
Non-absorbable Sutures are manufactured from special silk, or synthetic material like nylon, polyester, or poly propylene. Non-absorbable sutures are employed to close wounds on the skin, and may or may not include coatings to enhance performance characteristics. Non-absorbable sutures generally produce lower degrees of scarring which is why these are used in procedures where cosmetic outcomes are significant.
On the basis of suture material structure, a classification may be done. For example, a monofilament suture made of a single thread enables easy passage of the suture through the tissue. On the other hand, a braided suture comprising several small threads interwoven together offers more security, but has a greater potential for infections.
Sutures may also be classified on the basis of being manufactured from natural or synthetic material.
While sutures are employed to close wounds and expedite healing, there have been problems associated with their use, such as damage to soft tissues due to stiff fibres. Advancements in the domain are expected to lead to superior solutions and drive the global surgical sutures market. For example, researchers have devised an advanced tough gel sheathed (TGS) suture, based on the structure of the human tendon. The design of the solution is based on the endotenon sheath, which, due to its double-network structure, is strong and sturdy; it fastens collagen fibres together while its elastin network strengthens it. The next-generation sutures carry a slippery but strong gel envelop that imitates the structure of soft connective tissues. TGS sutures may be designed to offer personalized medicine on the basis of a patient's requirements.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Surgical Sutures Market Report and Forecast 2026-2035 offers a detailed analysis of the market based on the following segments:
By Type, the market is divided into
By Filament, the market is classified into
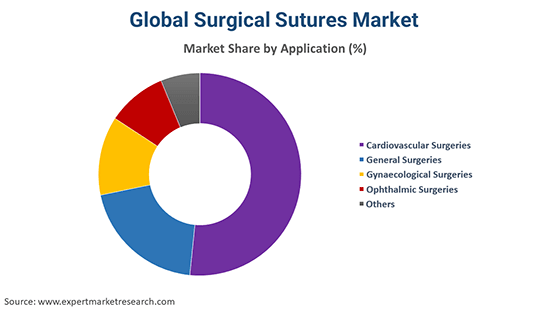
By Application, the market is segmented into
By Region, the market is classified into
The report presents a detailed analysis of the following key players in the global surgical sutures market, looking into their capacity, and latest developments like capacity expansions, plant turnarounds, and mergers and acquisitions:




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
The surgical sutures market was valued at USD 4.49 Billion in 2025.
The market is expected to grow at a CAGR of 6.00% from 2026 to 2035 to reach a value of USD 8.04 Billion by 2035.
The major drivers of the industry, such as the increased prevalence of chronic illnesses worldwide, growing geriatric population, heightened investments in the healthcare infrastructure, and increasing initiatives organised by international organisations to raise awareness pertaining to road accidents and injury treatments, are expected to aid the market growth.
The key market trends guiding the growth of the industry include the heightened prevalence of road accidents worldwide.
The major regions in the industry are North America, Latin America, the Middle East and Africa, Europe, and the Asia Pacific.
The significant products available in the market are automated suturing device and sutures.
The different materials considered within the market report include monofilament and multifilament.
The significant applications of the product include cardiovascular surgeries, general surgeries, gynaecological surgeries, ophthalmic surgeries, and others.
The major players in the industry are Braun Melsungen AG, Boston Scientific Corporation, ConMed Corporation, DemeTECH Corporation, Surgical Specialties Corporation, and others.
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
| REPORT FEATURES | DETAILS |
| Base Year | 2025 |
| Historical Period | 2019-2025 |
| Forecast Period | 2026-2035 |
| Scope of the Report |
Historical and Forecast Trends, Industry Drivers and Constraints, Historical and Forecast Market Analysis by Segment:
|
| Breakup by Product Type |
|
| Breakup by Filament Type |
|
| Breakup by Applications |
|
| Breakup by End User |
|
| Breakup by Region |
|
| Market Dynamics |
|
| Supplier Landscape |
|
| Companies Covered |
|
Datasheet
One User
USD 3,299
USD 2,969
tax inclusive*
Single User License
One User
USD 5,499
USD 4,949
tax inclusive*
Five User License
Five User
USD 6,999
USD 5,949
tax inclusive*
Corporate License
Unlimited Users
USD 8,199
USD 6,969
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Strategic Solutions for Informed Decision-Making
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share